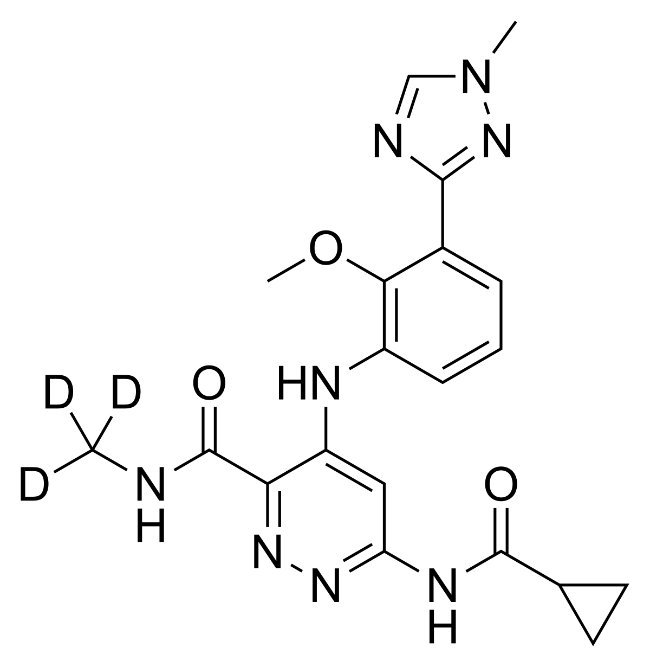
Deucravacitinib (FDA-approved on September 9, 2022)
A week ago, FDA-approved TYK2 inhibitor deucravacitinib (trade name Sotyktu) for moderate-to-severe plaque psoriasis and there are several reasons which make the news remarkable.
First of all, it is a successful final of a long story with this drug. The molecule was developed by BMS and its future was under question when the company acquired Celgene in 2019. Finally it was decided to continue its development and due to this decision BMS had to follow the requirement of the Federal Trade Commission and offload Celgene’s FDA-approved drug against psoriasis –PDE4 inhibitor Apremilast (trade name Otezla) which now belongs to Amgen. In 2021 deucravacitinib did not show impressive results in midphase ulcerative colitis study however next year it demonstrated solid efficacy in the treatment of plaque psoriasis compared to both placebo and Otezla. And now finally the drug is approved: BMS will get a new blockbuster and the patients – a new effective treatment for psoriasis.
On the other hand, deucravacitinib – is the first FDA-approved allosteric TYK2 inhibitor on the market. TYK2 is one of the first studied JAK family kinases. However, while a number of JAK-inhibitors are already on the market for the treatment of various autoimmune deceases, TYK2 inhibitors “delayed” due to some uncertainty regarding this therapeutic target. The success of deucravacitinib “inspired” other companies like Pfizer, Riovant, and Nimbus which have their own TYK2-inhibitors under development and intensified further studies. Thus hopefully we’ll see several more drugs from this family.
And finally, from the MedChem point of view, the news regarding deucravacitinib is remarkable because it is the second FDA-approved drug containing Deuterium atoms. The role of the deuterium isotope effect in drug metabolism and the perspective of deuterated drugs was discussed for many years in the literature however until now there was only one FDA-approved drug that contained Deuterium - VMAT2 inhibitor Deutetrabenazine (trade name Austedo) which was developed by Teva and approved in 2017 for the treatment of chorea associated with Huntington's disease and tardive dyskinesia.
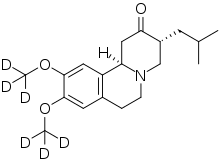
Deutetrabenazine – first FDA-approved deuterated drug
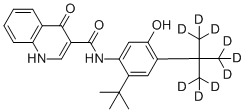
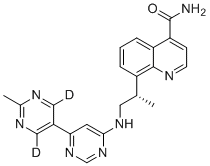
And now we have a “silver winner” in this “race” and this fact gives some additional confidence for the developers of deuterodrugs. There are several deuterated molecules in the clinic and of course it is interesting who will be the “bronze winner”. Perhaps it will be deuruxolitinib – the deuterated version of JAK-inhibitor ruxolitinib developed by Concert Pharmaceuticals? Or maybe deutivacaftor - the deuterium-modified form of ivacaftor from Vertex? It can be also deuterated fatty acid RT001 (Di-deuterated linoleic acid ethyl ester) which is under developmentby Retrotope for treatment of neurodegenerative diseases such as Friedreich's ataxia and infantile neuroaxonal dystrophy. And what about DNA-dependent protein kinase (DNA-PK) inhibitor VX-984 (M9831), discovered by Vertex (now belongs to Merck KGaA), containing Deuterium in a pyrimidine ring?..
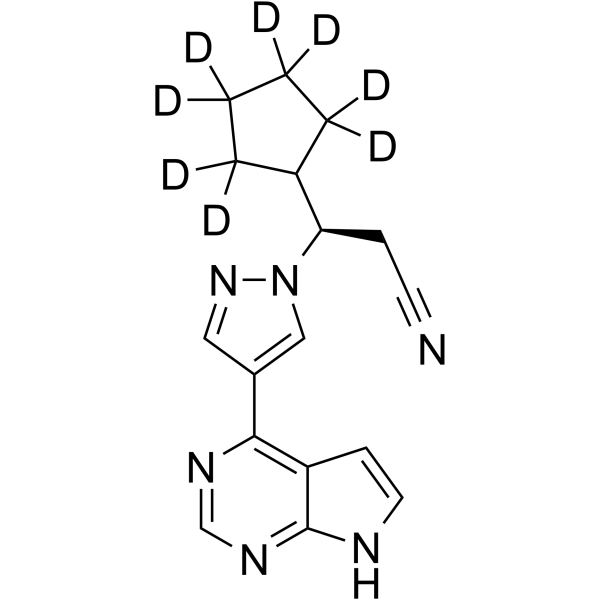
Structures of deuruxolitinib and deutivacaftor
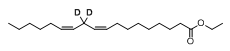
Structures of RT001 and VX-984 (M9831)
It will be interesting to follow the future of these and other deuterated molecules. In any way the introduction of Deuterium into active molecules is a sustainable trend in Drug Discovery. Understanding this we at Enamine enlarge our collection of Building Blocks with deuterium-containing molecules and hope they will help for future endeavors in this fascinating area.
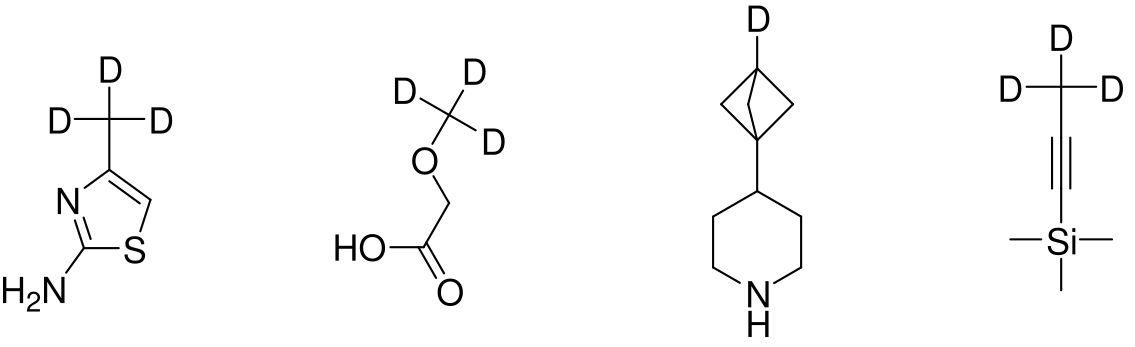
Examples of Enamine deuterated Building Blocks
