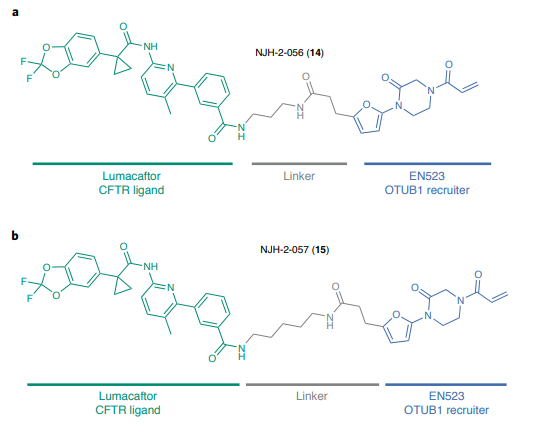
Structures of first DUBTACs (from Nature Chemical Biology volume 18, pages 412–421, 2022)
Few weeks ago a new biotech appeared on the map: Vicinitas Therapeutics launched with $65 Million in Series A. The company is focused on developing small-molecule drugs that stop the degradation of specific proteins to restore their levels for therapeutic benefit. The main value and basis of the biotech is the research of Daniel Nomura’s lab at UC Berkeley on so-called Deubiquitinase-targeting chimeras (or DUBTACs) for targeted protein stabilization.
Daniel Nomura, Vicinitas Therapeutics founder
It is really interesting area of research which is close but at the same time opposite to other hot topic in modern Drug Discovery – PROTACs. Both PROTACs and DUBTACs are bifunctional molecules with two active “anchors” connected by flexible linkers. However, their behavior in the cells are absolutely different.
PROTACs bring together the enzyme called E3 ligase and the targeted protein. That leads to “labeling” of the protein with ubiquitin and further degradation by cell machinery (known as Ubiquitin-Proteasome System or UPS). At the same time DUBTACs brings together the targeted protein and deubiquitinase – the enzyme which function is to remove ubiquitin “labels”. This “vicinity” of both proteins (that’s where the name Vicinitas comes from) prevents degradation of the protein by UPS and as a result it remains in the cell and continue to operate.
The proof of concept for DUBTACs was recently published by Daniel Nomura’s lab in the Nature Chemical Biology. In this paper the team first find out a small covalent ligand EN523 that targets a non-catalytic allosteric cysteine C23 in the K48-ubiquitin-specific deubiquitinase OTUB1.
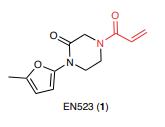
Structure of EN523 (from Nature Chemical Biology volume 18, pages 412–421, 2022)
Next the first DUBTACs were designed and synthesized. And the first aim was to stabilize cystic fibrosis transmembrane conductance regulator (CFTR) – well investigated target for the treatment of cystic fibrosis especially in the case of ΔF508 mutation. Having such a mutation the protein degrades quickly in the cells and its lack leads to the decease development. During last several years several drugs from Vertex were approved for the treatment of cystic fibrosis targeted CFTR in order to stabilize it. And one of such drugs targeted ΔF508-CFTR – lumacaftor was used for the design of these new DUBTACs. Thus, the final molecule is a “dumbbell” with the motif of EN523 on the one side and lumacaftor fragment on the other side. And they’re connected by the flexible linkers. One side binds to deubiquitinase, another one binds to ΔF508-CFTR, it brings deubiquitinase close to ΔF508-CFTR and “motivate” deubiquitinase to “sweep out” ubiquitin from ΔF508-CFTR once it is labeled.
And this moleculeі really work! They robustly stabilized ΔF508-CFTR protein levels in the cells!
Exactly these promising results induced the launch of Vicinitas Therapeutics and the company will definitely dive deeply in this amazing area to create the real drugs.
But let’s come back to the very first steps of this journey, to the research of small molecule binders of deubiquitinase OTUB1 which precedes the first DUBTAC creation… What does it start from?
If you go to the paper and take a look you can easily find that the project started from screening of cysteine covalent ligand libraries, including the compounds purchased from Enamine. Actually, the identified ligand of OTUB1 EN523 is compound from Enamine collection.
And it is really amazing to see how this small covalent molecule synthesized in Ukraine several years ago initiated the intensive research in a new perspective area and launching the biotech in the other part of the world!
Best wishes to Professor Nomura, his lab and the whole team of Vicinitas in their endeavors! It is going to be really exciting adventure to develop DUBTACs against some “undruggable” targets!
From our end we continue to support academia and biotech/pharma companies with our high-quality products and services. If you have your own (non-confidential) story how Enamine molecules helped in your research, please contact us and we’ll be glad to share it in our blog.
