Eur. J. Org. Chem. 2019, (22), 3553-3559
DOI: 10.1002/ejoc.201801750
Fominova K.; Diachuk T.; Sadkova I.; Mykhailiuk P.
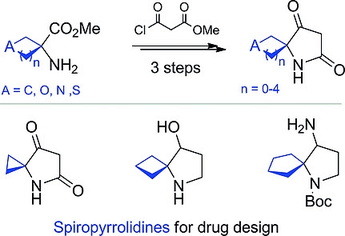
Novel spirocyclic pyrrolidines were synthesized in three steps from cyclic α‐amino acids with 3‐ to 7‐membered cycle. The key transformation was the Dieckmann condensation reaction. The described approach opens a door to various novel spirocyclic building blocks for drug design.