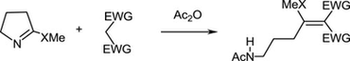Tetrahedron 2015, 71 (40), 7567-7574
DOI: 10.1016/j.tet.2015.08.009
Shvydenko K.; Nazarenko K.; Shvydenko T.; Vlasenko Y.; Tolmachev A.; Kostyuk A.
The reaction of cyclic thioimidates with active methylene compounds in the presence of acetic acid anhydride was studied. In case of six- and seven-membered derivatives the reaction proceeded via substitution of thiomethoxy group affording cyclic enamines. In case of five-membered derivatives the reaction proceeded via opening of the thioimidate ring affording functionalized aminopropane derivatives.