Tetrahedron , 2013, 69 (2), 505-511
DOI: 10.1016/j.tet.2012.11.032
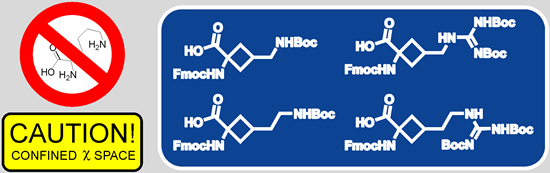
Four χ1,χ2-constrained cyclobutane-derived basic amino acids—conformationally restricted analogues of arginine, lysine and ornithine—were prepared as the derivatives properly protected for Fmoc-solid phase peptide synthesis. Compatibility of the synthesized arginine analogues with standard procedures of the Fmoc solid-phase peptide synthesis was demonstrated by incorporating these residues into the small cyclic antimicrobial peptide c-(RRRWFW) substituting the arginine residues. These replacements did not affect much the antimicrobial activity of the peptides.
Radchenko D. S.; Michurin O. M.; Grygorenko O. O.; Scheinpflug K.; Dathe M.; Komarov I. V.
Tetrahedron 2013, 69 (2), 505-511
DOI: 10.1016/j.tet.2012.11.032
