Phosphorus, Sulfur Silicon Relat. Elem. 2018, 193 (7), 409-414
DOI: 10.1080/10426507.2018.1452236
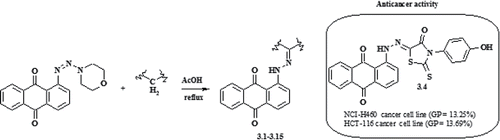
A series of polyfunctionalized anthraquinonehydrazones have been prepared using azo-coupling reaction between anthraquinone-based triazenes and methylene active compounds in acetic acid without a catalyst. The structures of new synthesized compounds were confirmed by their IR, LCMS,1H and 13C NMR spectroscopic data. Some of the synthesized compounds were screened for their in vitro anticancer activity according to US NCI protocols. Biological screening data led to the identification of 1-{N’-[3-(4-hydroxyphenyl)-4-oxo-2-thioxothiazolidin-5-ylidene]hydrazino}anthraquinone 3.4 as having antitumor activity on the non-small cell lung cancer NCI-H460 (GP = 13.25%) and colon cancer HCT-116 (GP = 13.69%) cell lines.