J. Fluorine Chem. 2018, 211, 100-108
DOI: 10.1016/j.jfluchem.2018.03.014
Kondratov I.; Bugera M.; Tolmachova N.; Daniliuc C. Haufe G.
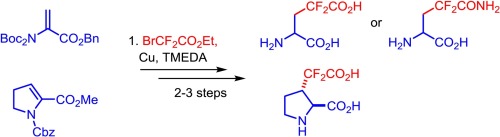
Copper-mediated Michael addition of ethyl bromodifluoroacetate to N-protected α,β-unsaturated α-amino acid esters was applied for straightforward synthesis of γ,γ-difluorinated analogues of glutamic acid (compound 1) and glutamine (compound 10). Moreover, a proline-based, sterically constrained analog of γ,γ-difluoroglutamic acid (compound 24) was prepared.