Monatsh. Chem. 2017, 148 (5), 939-946
DOI: 10.1007/s00706-016-1884-6
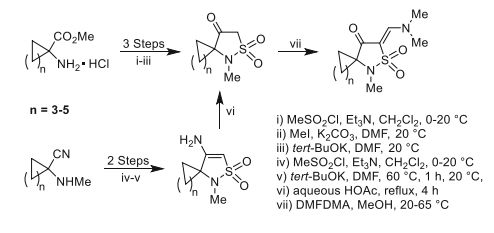
We have introduced a strategy for the construction of spirocycloalkane 1λ6-isothiazolidine-1,1,4-triones through the mesylation of 1-aminocyclopentane-, 1-aminocyclohexane-, and 1-aminocycloheptanecarboxylic acid esters with methanesulfonylchloride followed by alkylation with methyl iodide and consequent cyclization in the presence of potassium tert-butoxide in N,N-dimethylformamide. The spirocycloalkane 4-amino-2,3-dihydro-1H-1λ6-isothiazole-1,1-diones were prepared via mesylation of N-methylated 1-aminocyclopentyl-, 1-aminocyclohexyl-, and 1-aminocycloheptyl carbonitriles followed by treatment of obtained N-(1-cyanocycloalkyl)-N-methylmethanesulfonamides with potassium tert-butoxide in N,N-dimethylformamide. The spiro 4-amino-2,3-dihydro-1H-1λ6-isothiazole-1,1-diones were converted into the target spiro 1λ6-isothiazolidine-1,1,4-triones by acid-catalyzed hydrolysis. The structure of a target spiro compound and its isolated key intermediate was confirmed by X-ray diffraction study. The interaction of spiro 1λ6-isothiazolidine-1,1,4-triones with N,N-dimethylformamide dimethyl acetal leads to the formation of spiro 5-[(Z)-(dimethylamino)methylidene]-1λ6-isothiazolidine-1,1,4-triones.