Asian J. Org. Chem. 2016, 5 (4), 513-520
DOI: 10.1002/ajoc.201500519
Khutoryanskiy V. V.; Biitseva A. V.; Mykhailiuk P. K.
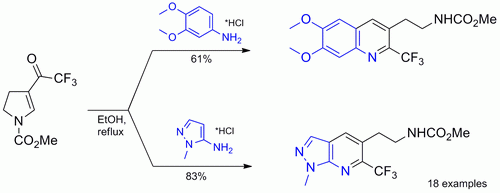
Electron-rich anilines and amino heterocycles were converted in one step into N-protected functionalized aminoethyl 2-trifluoromethylquinolines and their heteroaromatic analogues. The N-carbamate protecting group can be easily cleaved under acidic conditions to give the corresponding amines.