Tetrahedron: Asymmetry 2015, 26 (2122), 1268-1272
DOI: 10.1016/j.tetasy.2015.09.015
Tymtsunik A. V.; Ivon Y. M.; Komarov I. V.; Grygorenko O. O.
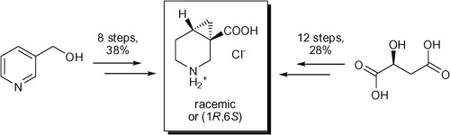
The synthesis of both racemic and enantiomerically pure (1R,6S)-3,4-methanonipecotic acid, a cyclopropane-containing β-amino acid, which is a valuable building block for drug discovery, is described. The synthetic scheme commences from natural (S)-malic acid and allows for the preparation of the title compound in 12 steps in 28% overall yield. A novel approach to the racemic 3,4-methanonipecotic acid, which relies on a Simmons–Smith cyclopropanation as the key step, was also developed. In this case, the product was obtained in 8 steps and 38% total yield.