Beilstein J. Org. Chem. 2015, 11 16-24
DOI: 10.3762/bjoc.11.3
Mykhailiuk P. K.
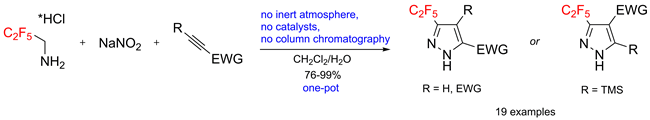
A one-pot reaction between C2F5CH2NH2•HCl, NaNO2 and electron-deficient alkynes gives C2F5-substituted pyrazoles in excellent yields. The transformation smoothly proceeds in dichloromethane/water, tolerates the presence of air, and requires no purification of products by column chromatography. Mechanistically, C2F5CH2NH2•HCl and NaNO2 react first in water to generate C2F5CHN2, that participates in a [3 + 2] cycloaddition with electron-deficient alkynes in dichloromethane.