ACS Comb. Sci. , 2014, 16 (3), 146-153
DOI: 10.1021/co4001277
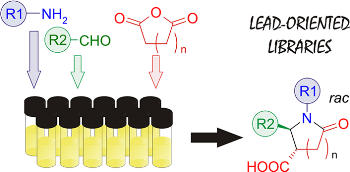
One-pot variation of Castagnoli condensation, that is, reaction of cyclic anhydrides, amines, and aldehydes, has been developed as a combinatorial approach to 1,2-disubstituted 5-oxopyrrolidine- and 6-oxopiperidine-3-carboxylic acids, as well as their benzo-analogues. Utility of the method to multigram preparation of building blocks and synthetic intermediates was also demonstrated. The final products are obtained in high yields and diastereoselectivity. The method fits well in the concept of lead-oriented synthesis; in particular, it can be used for the design of lead-like compound libraries, even if the strictest cut-offs are applied to the physicochemical properties of their members.
Ryabukhin S. V.; Panov D. M.; Granat D. S.; Ostapchuk E. N.; Kryvoruchko D. V.; Grygorenko O. O.
ACS Comb. Sci. 2014, 16 (3), 146-153
DOI: 10.1021/co4001277
