Angew. Chem. Int. Ed. , 2014, 53 (13), 3392‑3395
DOI: 10.1002/anie.201310019
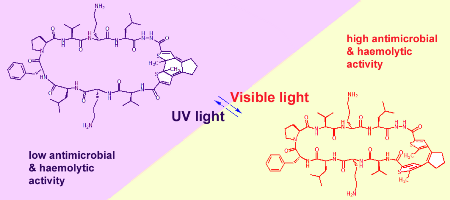
Photobiological processes in nature are usually triggered by nonpeptidic chromophores or by modified side chains. A system is presented in which the polypeptide backbone itself can be conformationally switched by light. An amino acid analogue was designed and synthesized based on a reversibly photoisomerizable diarylethene scaffold. This analogue was incorporated into the cyclic backbone of the antimicrobial peptide gramicidin S at several sites. The biological activity of the resulting peptidomimetics could then be effectively controlled by ultraviolet/visible light within strictly defined spatial and temporal limits.
Babii O.; Afonin S.; Berditsch M.; Reiβer S.; Mykhailiuk P. K.; Kubyshkin V. S.; Steinbrecher T.; Ulrich A. S.; Komarov I. V.
Angew. Chem. Int. Ed. 2014, 53 (13), 3392-3395
DOI: 10.1002/anie.201310019
