Org. Biomol. Chem. , 2013, 11 (6), 975-983
DOI: 10.1039/C2OB27058G
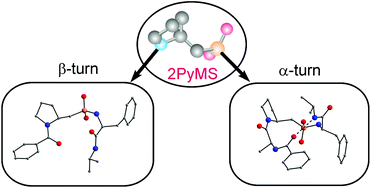
The conformational behaviour of model peptides containing a 2-pyrrolidinemethanesulfonic acid (2PyMS) residue was studied in both the crystalline state and in solution using X-ray, NMR and IR experiments. It was found that in crystals dipeptide PhC(O)-2PyMS-Phe-NHiPr adopted [small beta]-turn conformation, which was not stabilized by an intramolecular hydrogen bond and could be classified as a type IV β-turn. In the crystalline state tripeptide PhC(O)-Ala-2PyMS-Phe-NHiPr existed as an α-turn with uncommon cis-conformation of the amide bond formed by the pyrrolidine nitrogen atom of the 2PyMS residue, for which no close analogue can be envisaged among the tight turns identified so far. Although the tendency to adopt folded conformations was only partially retained in solution, 2-PyMS could be considered as a promising structural unit for the design of foldamers and peptidomimetics with unusual conformational properties.
Grygorenko O. O.; Zhersh S.; Oliinyk B. V.; Shishkin O. V.; Tolmachev A. A.
Org. Biomol. Chem. 2013, 11 (6), 975-983
DOI: 10.1039/C2OB27058G
