Tetrahedron , 2011, 67 (17), 3091-3097
DOI: 10.1016/j.tet.2011.02.082
Mykhailiuk P. K.; Shishkina S. V.; Shishkin O. V.; Zaporozhets O. A.; Komarov I. V.
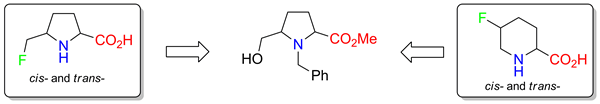
The synthesis of proline analogues bearing a fluorine-containing substituent at the fifth position of the pyrrolidine ring, racemic trans- and cis-5-fluoromethyl prolines, was performed. The key step of the synthesis is a transformation of the CH2OH-group into the CH2F-one using morpholinosulfur trifluoride. During the synthesis, an efficient procedure to prepare trans- and cis-5-fluoropipecolic acids was elaborated.
Mykhailiuk P. K.; Shishkina S. V.; Shishkin O. V.; Zaporozhets O. A.; Komarov I. V.
Tetrahedron 2011, 67 (17), 3091-3097
DOI: 10.1016/j.tet.2011.02.082
