Tetrahedron: Asymmetry , 2010, 21 (23), 2868-2871
DOI: 10.1016/j.tetasy.2010.11.017
Kopylova N. A.; Grygorenko O. O.; Komarov I. V.; Groth U.
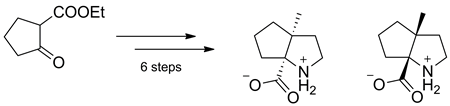
A novel approach to the synthesis of an enantiopure bicyclic proline analogue, hexahydrocyclopenta[b]pyrrole-6a(1H)-carboxylic acid (‘2,3-propanoproline’), has been developed. The method relied on tandem Strecker-nucleophilic cyclization reaction of 2-(2-bromoethyl)cyclopentanone. The overall synthetic scheme included six steps and resulted in 18% overall yield of both enantiomers of the title amino acid.
Kopylova N. A.; Grygorenko O. O.; Komarov I. V.; Groth U.
Tetrahedron: Asymmetry 2010, 21 (23), 2868-2871
DOI: 10.1016/j.tetasy.2010.11.017
