Synthesis , 2009, 19, 3233-3242
DOI: 10.1055/s-0029-1216957
Kotljarov A.; Irgashev R. A.; Iaroshenko V. O.; Sevenard D. V.; Sosnovskikh V. Y.
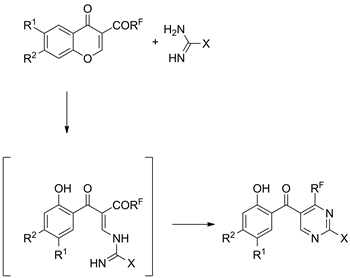
Reactions of 3-(polyfluoroacyl)chromones and their hetero analogues with a number of 1,3-NCN-dinucleophiles, such as amidines or guanidines, were studied in detail, and preparative access to a set of diverse 5-salicyloyl-4-(polyfluoroalkyl)pyrimidines was elaborated. These compounds appear to be a suitable starting substrates for the synthesis of 4-(polyfluoroalkyl)pyrimidine-5-carboxylic acids or pyrimidines containing a benzofuran-3-yl substituent in the 5-position.
Kotljarov A.; Irgashev R. A.; Iaroshenko V. O.; Sevenard D. V.; Sosnovskikh V. Y.
Synthesis 2009, 19, 3233-3242
DOI: 10.1055/s-0029-1216957
