Chem. Heterocycl. Compd. 2020, 56 (3), 377–385
DOI: 10.1007/s10593-020-02670-z
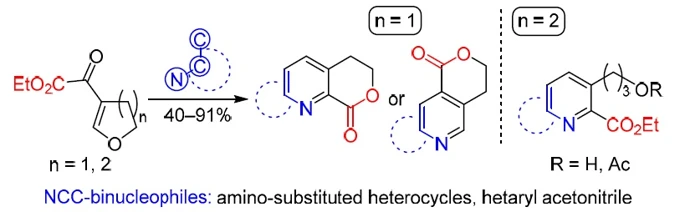
Reactions of five- and six-membered cyclic β-alkoxyvinyl α-keto esters and NCC-binucleophiles are described. The following binucleophiles were studied: heteroaromatic amines (pyrazoles, isoxazole, uracils, and isoquinolinone) and 2-(benzimidazolyl)acetonitrile. It was found that condensation proceeded regioselectively in the case of five-membered cyclic enone. Fused α-pyridine carboxylates, likely formed in situ, underwent lactonization, which resulted in six-membered lactones in moderate to excellent yields (53–91%). Only in the case of 3-unsubstituted 5-aminopyrazole, formation of γ-pyridine carboxylate followed by cyclization to isomeric lactone was observed. On the contrary, six-membered cyclic enone was reactive only toward heterocyclic amines under optimized conditions and provided open-chain α-pyridine carboxylates in 40–80% yield.