Chem. Heterocycl. Compd. 2019, 55 (4-5), 359-366
DOI: 10.1007/s10593-019-02465-x
Petko K.; Sokolenko T.; Filatov A.; Polovinko V.; Rusanov E.; Dudko V.; Yagupolskii Y.
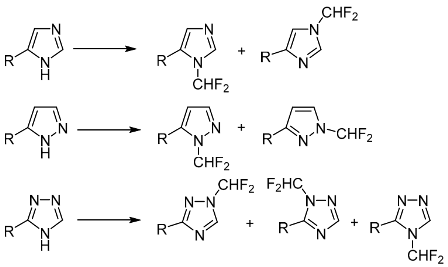
This study was focused on N-difluoromethylation of monosubstituted polydentate azoles (imidazoles, pyrazoles, and 1,2,4-triazoles) having two or three reactive sites. The substituent effects and role of reaction conditions in determining the product ratio was explored. In the majority of cases, the obtained mixtures of isomers were separated. The target products containing halogen substituents, amino or carboxy groups in the ring can serve as valuable starting compounds in the synthesis of practically useful products.