Eur. J. Org. Chem. 2019 (22), 3636-3648
DOI: 10.1002/ejoc.201900450
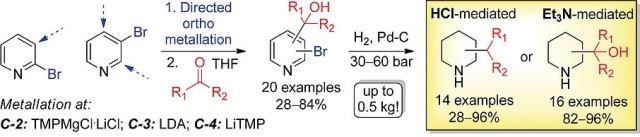
An efficient approach towards introducing (cyclo)alkyl substituents at C‐2, C‐3 or C‐4 positions of the piperidine ring was described. The method relied on the straightforward two‐step reaction sequence based on the formal sp3–sp3 retrosynthetic disconnection. The procedure commenced with selective directed ortho metalation of 2‐ and 3‐bromopyridine, followed by reaction with aldehydes or ketones. The optimized methods were developed for all three isomers of hydroxyalkyl‐substituted pyridines, which were synthesized in 28–84 % overall yield (20 examples). Catalytic hydrogenation of these adducts could be performed selectively with or without retention of the hydroxyl group in their molecules, so that either (cyclo)alkylpiperidines (14 examples) or the corresponding saturated amino alcohols (16 examples) were obtained (28–96 % and 82–96 % yield, respectively). After minor modifications, the developed method was also implemented in a flow reactor and a 5 L autoclave, which allowed for the preparation of up to 0.5 kg of the representative (cyclo)alkylpiperidines.