New J. Chem. 2018, 42 (16), 13461-13470
DOI: 10.1039/c8nj02631a
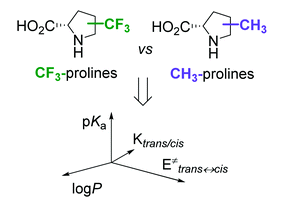
Proline is one of a kind. This amino acid exhibits a variety of unique functions in biological contexts, which continue to be discovered and developed. In addition to the reactivity of the primary functional groups, the trans–cis isomerization of the peptidyl–prolyl amide bond and its impact on the protein structure and function are of major interest. A variety of proline ring substitutions occur in nature, and more substitutions have been generated via chemical synthesis. Particularly promising is the 19F-labelling of proline, which offers a relatively new research application area. For example, it circumvents the lack of common NH-NMR reporters in peptidyl–prolyl fragments. Obtaining structural information from selectively fluorine-labelled peptides, proteins, and non-peptidic structures requires the analysis of the physicochemical features of the 19F-carrying proline analogues. To better understand and ultimately predict the potential perturbations (e.g., in protein stability and dynamics) introduced by fluorine labels, we conducted a comprehensive survey of the physicochemical effects of CF3 substitutions at each ring position by comparing the behavior of CF3-substituted residues with the CH3-substituted analogues. The parameters analyzed include the acid–base properties of the main chain functional groups, carbonyl-group interaction around the residue, and the thermodynamics and kinetics of trans–cis isomerization. The results reveal significant factors to consider with the use of CF3-substituted prolines in NMR labeling and other applications. Furthermore, lipophilicity measurements demonstrate that CF3-substituted proline shows comparable hydrophobicity to valine, suggesting the potential application of these residues for enhancing interactions at nonpolar interfaces.