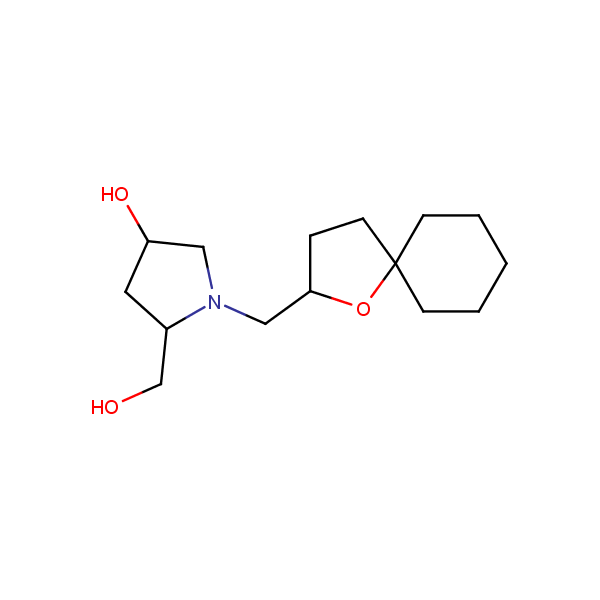
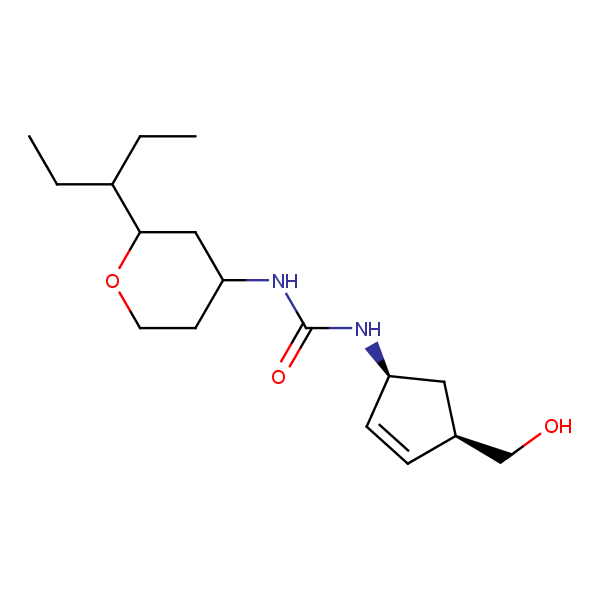
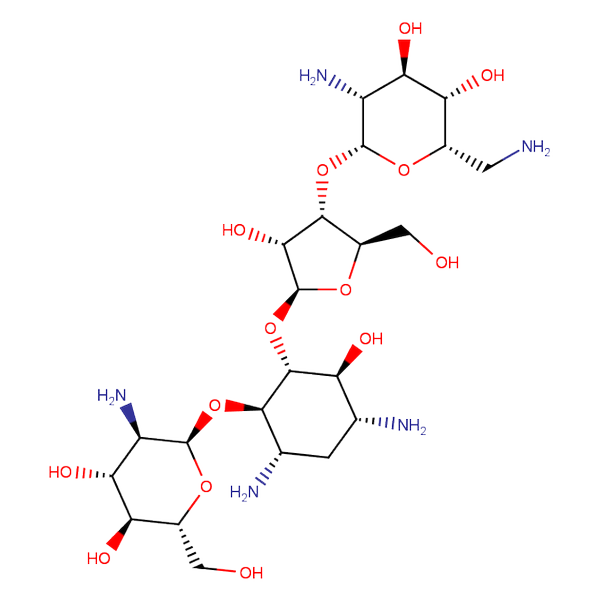
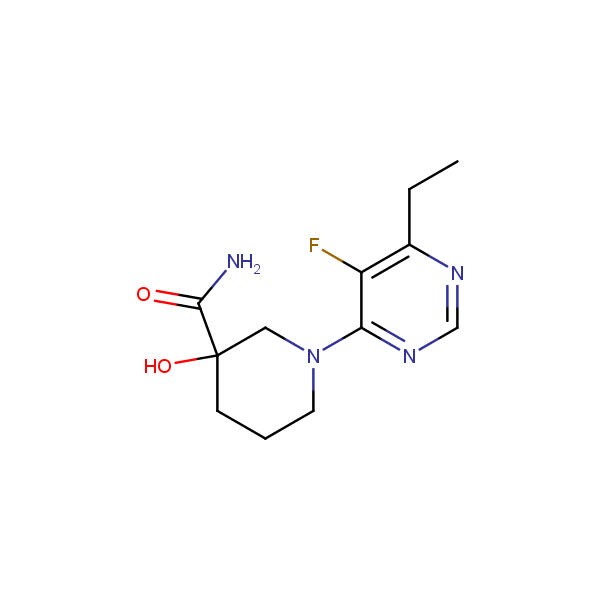
Specially synthesized set of compounds able to mimic glycosides and their interaction with proteins
2 470 compounds
The main emphasis in the library design was made on drug-like compounds enriched with H-bond donors (possess at least two H-bond donors) and bearing nature-like Fsp3–rich scaffolds with diverse spatial orientation of H-bond donors and different 3D-shapes. We suppose the library application would identify new attractive chemotypes which correspond to drug-/lead-like criteria and on the other hand participate in key interactions similar to glycoside binding in the active site of the targets.
Typical Formats
Glycomimetic Library is available for supply in various pre-plated formats, including the following most popular ones:
Catalog No.
GML-2470-0-Z-10
Compounds
2 470
2 plates
Amount
≤ 300 nL of 10 mM of DMSO solutions
Plates and formats
1536-well Echo LDV microplates, first and last four columns empty, 1280 compounds per plate
Price
Catalog No.
GML-2470-10-Y-10
Compounds
2 470
8 plates
Amount
≤ 10 µL of 10 mM DMSO solutions
Plates and formats
384-well, Echo Qualified LDV microplates #001-12782 (LP-0200), first and last two columns empty, 320 compounds per plate
Price
Catalog No.
GML-2470-50-Y-10
Compounds
2 470
8 plates
Amount
50 μL of 10 mM DMSO solutions
Plates and formats
384-well, Greiner Bio-One plates #781280, 1,2 and 23,24 columns empty, 320 compounds per plate
Price
Catalog No.
Library & follow-up package
Plates and formats
GML-2470-10-Y-10 screening library 2 470 cmpds, hit resupply, analogs from 4.6M+ stock and synthesis from REAL Space
Price
*We will be happy to provide our library in any other most convenient for your project format. Please select among the following our standard microplates: Greiner Bio-One 781270, 784201, 781280, 651201 or Echo Qualified 001-12782 (LP-0200), 001-14555 (PP-0200), 001-6969 (LP-0400), C52621 or send your preferred labware. Compounds pooling can be provided upon request.
Download SD files
Library design
- Privileged core fragments and side chain functionalities:
- cyclic amino alcohols, cyclic glycols
- O,N-containing aliphatic rings
- polyalcohols
- Nature-likeness scoring algorithm: Taverna 2.5 workbench, similarity to available NP databases
- MedChem structural filters, removal of common and trivial chemotypes: PAINS, REOS, Elli Lily rules and strict Enamine filters
Training set of known glycosides was collected from the public available e-databases, scientific literature and evaluated to define the optimal range of molecular parameters.