New original ligands are based on chiral natural terpenoids, mostly on camphor. Some examples of the ligands we can synthesize are shown below:
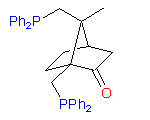
1
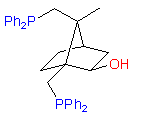
2
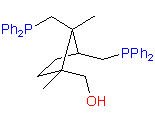
catASium I©
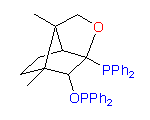
3
The hydroxydiphosphine ligand catASium I© and phosphine-phosphinite 3 are produced in collaboration with Degussa (patent application WO 03/099532). The compounds can be used for research purposes only and commercial quantities (5 g to several kg) are available from Degussa only (www.degussa-catalysts.com).
All the ligands are of >90% chemical purity and >99% optical purity.
• Chiral aminoalcohols used as chiral auxiliaries or as ligands for asymmetric catalysts (see Comprehensive Asymmetric Catalysis, (Eds.: E. N. Jacobsen, A. Pfaltz, H. Yamamoto), Springer, Heidelberg, 1999, Vol. I-III). We develop syntheses of new aminoalcohols starting from chiral pool, examples are shown below:
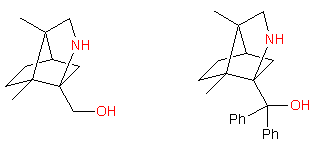
The aminoalcohols are of >95% chemical purity and >99% optical purity. Up to 2 g of each compound could be synthesized.
