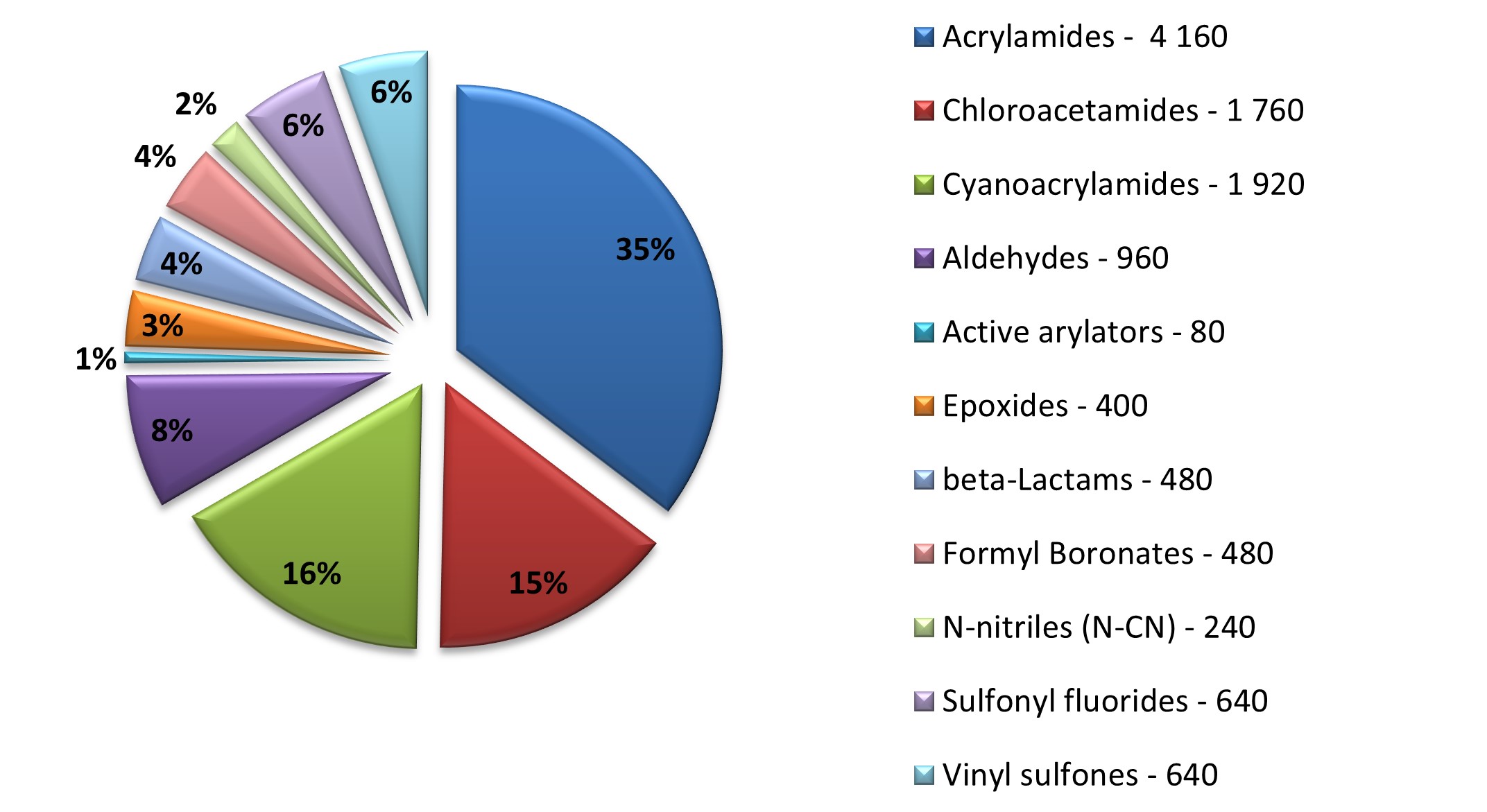
Diverse covalent library with most demanded warhead types
11 760 compounds
Covalent probes play an essential role in the discovery of new technologies, investigation of new proteins, and assessment of their drugability. We at Enamine have been working since 2016 on synthesis on covalent compounds and development of new covalent warheads.
Enamine focused on the elaboration of parallel synthesis approaches to synthesize a series of new valuable covalent compounds with well-working and not overreactive warheads. All our efforts resulted in the production of the largest commercially available Collection of Covalent Compounds. Most interesting and popular covalent classes were assembled into a diverse Library, aimed to represent Enamine’s Covalent Collection.
The Library is available in pre-plated format at 10 mM concentration in DMSO. Each covalent binder type is plated in separate plates.
Typical Formats
Covalent Screening Library is available for supply in various pre-plated formats, including the following most popular ones:
Catalog No.
Covalent Screening Library
CSL-11-10-Y-10
Compounds
11 760
37 plates
Amount
10 µL of 10 mM DMSO solutions
Plates and formats
384-well, Echo Qualified LDV microplates #001-12782 (LP-0200), first and last two columns empty, 320 compounds per plate
Price
Catalog No.
Representative Set
CSRS-320-20-Y-10
Compounds
320
1 plate
Amount
20 μL of 10 mM DMSO solutions
Plates and formats
384-well Echo Qualified LDV plates, 1, 2 and 23, 24 columns empty, 320 compounds per plate
Price
Catalog No.
Acrylamides
CSAC-4160-20-Y-10
Compounds
4 160
13 plates
Amount
20 μL of 10 mM DMSO solutions
Plates and formats
384-well plates, Greiner #765021, 1, 2 and 23, 24 columns empty, 320 compounds per plate
Price
Catalog No.
Cyanacrylamides
CSCN-1920-10-Y-10
Compounds
1 920
6 plates
Amount
10 µL of 10 mM DMSO solutions
Plates and formats
384-well echo LDV plates, #LP-200, 1,2 and 23,24 columns empty, 320 compounds per plate
Price
Catalog No.
Chloroacetamides
CSCL-1200-25-Y-10
Compounds
1 200
4 plates
Amount
25 μL of 10 mM DMSO solutions
Plates and formats
384-well plates, Greiner #784201, 1, 2 and 23, 24 columns empty, 320 compounds per plate
Price
Catalog No.
Vinyl Sulfones
CSVS-640-50-X-10
Compounds
640
2 plates
Amount
50 µL of 10 mM DMSO solutions
Plates and formats
96-well plates, 2D-barcoded Matrix microtubes #4271, 1 and 12 columns empty, 80 compounds per plate
Price
Catalog No.
Formyl Boronates
FBA-480-15-Y-10
Compounds
480
2 plates
Amount
15 µL of 10 mM DMSO solutions
Plates and formats
384-well echo plates, Labcyte #PP-0200, 1,2 and 23,24 columns empty, 320 compounds per plate
Price
*We will be happy to provide our library in any other most convenient for your project format. Please select among the following our standard microplates: Greiner Bio-One 781270, 784201, 781280, 651201 or Echo Qualified 001-12782 (LP-0200), 001-14555 (PP-0200), 001-6969 (LP-0400), C52621 or send your preferred labware. Compounds pooling can be provided upon request.
Download SD files
Key features
- Careful choice of warheads: only experimentally confirmed, well-validated, and reported in the number of papers. No overreactive binders.
- All compounds in the library feature a certain chemotype, “recognition pattern”, to avoid promiscuous bindings.
- Compounds plated by classes for ease of reactivity analysis and residue-focusing screening campaigns.
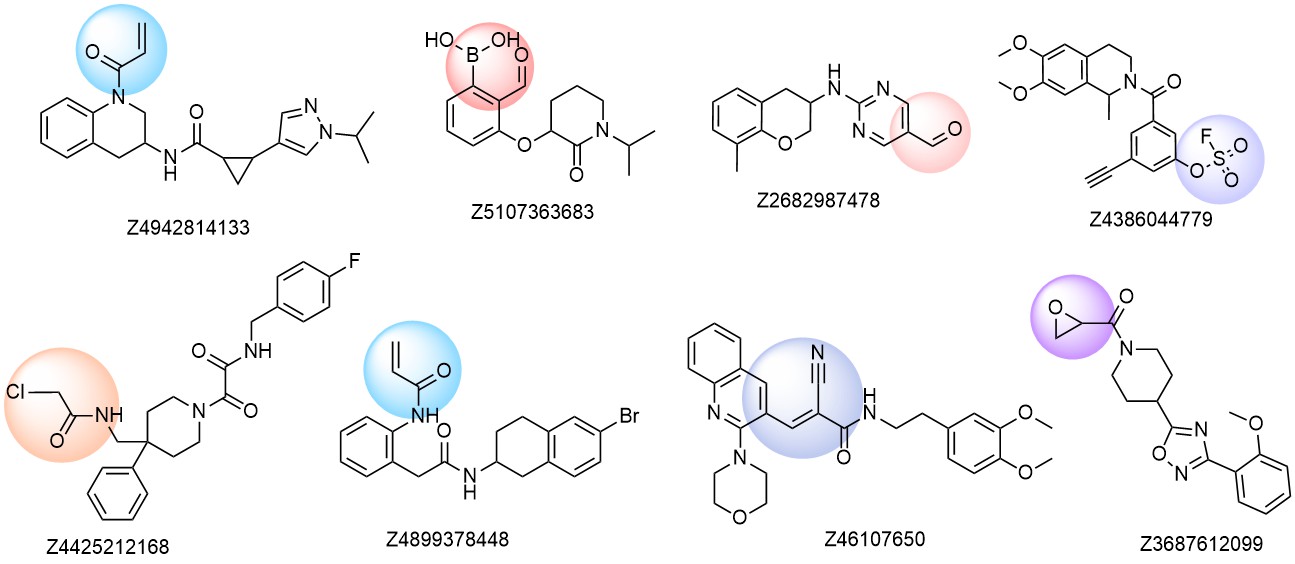
Examples of molecules in the library
Distribution by covalent warheads