CAS 100045-83-8, Cat. No EN300-18631891
Reagent for synthesis of MOM-protected alcohols
![Tributyl[(methoxymethoxy)methyl]stannane](/images/Reagents/EN300-18631891_Scheme.jpg)
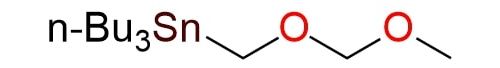
Tributyl[(methoxymethoxy)methyl]stannane (Sn-MOM) is a highly versatile reagent used in organic synthesis primarily for the protection of alcohols as methoxymethyl (MOM) ethers and the formation of 1,2-diols1. The reagent undergoes tin-lithium exchange with n-BuLi to generate an organolithium intermediate, a hydroxymethyl anion equivalent2. The intermediate reacts with carbonyl compounds, including aldehydes, ketones, and lactones, yielding mono-protected diols under mild conditions. The reagent reacts with Weinreb amides to form protected hydroxyketones while maintaining the stereochemistry of the compound3. Sn-MOM is stable and can be stored in a freezer under nitrogen for several months.
Synonyms: tributyl[(methoxymethoxy)methyl]stannane (ACI); (methoxymethoxymethyl)tributyltin; [(methoxymethoxy)methyl]tributylstannane; Sn-MOM
Selected publications
-
Tributyl[(Methoxymethoxy)Methyl]Stannane.
Romines K. Encyclopedia of Reagents for Organic Synthesis 2001. DOI: 10.1002/047084289X.rt172
-
A Hydroxymethyl Anion Equivalent: Tributyl[(Methoxymethoxy)Methyl]Stannane.
Organic Syntheses 1993, 71, 133. DOI: 10.15227/orgsyn.071.0133
-
Synthesis of Chiral Hexynones for Use as Precursors to Native Photosynthetic Hydroporphyrins.
Chau Nguyen K.; Chung D. ; Nalaoh P.; Lindsey J. Organic Syntheses 2024, 48 (5), 2097–2117. DOI: 10.1039/D3NJ03900E