CAS 10000-20-1, Cat. No EN300-25465329
Reagent source of hydrazine
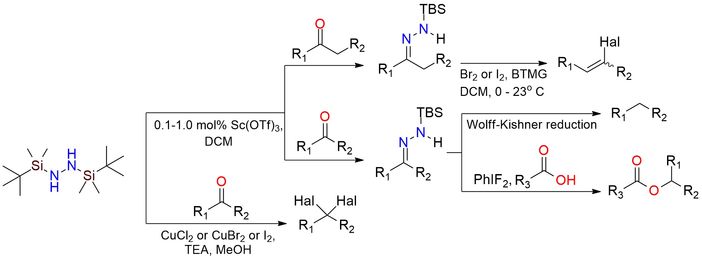
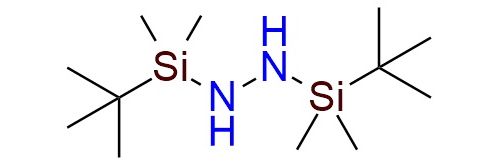
1,2-Bis(tert-butyldimethylsilyl)hydrazine (BTBSH) is a stable hydrazine derivative reagent used extensively in organic synthesis1. It’s found extensive usage in the preparation of N-silyl hydrazones, which serve as intermediates in Wolff-Kishner reductions2, gem-dihalide and vinyl halide synthesis under ambient or low-temperature conditions. BTBSH is a colorless liquid with excellent stability under inert, moisture-free conditions and can be stored for extended periods. The reagent demonstrates high compatibility with various functional groups, including acid- and base-labile moieties, enabling its application in sensitive substrates. There is a methodology using BTBSH for efficient esterification of carboxylic acids through in situ diazoalkane generation, providing high yields under mild and safe conditions3.
Synonyms: 1,2-bis-(tert-butyldimethylsilyl)hydrazine; 1,2-bis[tert-butyl(dimethyl)silyl]hydrazinel; hydrazine, 1,2-bis[(1,1-dimethylethyl)dimethylsilyl]-; N-t-butyldimethylsilylhydrazone; BTBSH
Selected publications
-
1,2-Bis(t-Butyldimethylsilyl)Hydrazine.
Filzen G. Encyclopedia of Reagents for Organic Synthesis 2007. DOI: 10.1002/047084289X.rn00759
-
Practical Procedures for the Preparation of N-tert-Butyldimethylsilylhydrazones and Their Use in Modified Wolff−Kishner Reductions and in the Synthesis of Vinyl Halides and gem-Dihalides.
Furrow M.; Myers A. J Am Chem Soc 2004, 126 (17), 5436–5445. DOI: 10.1021/ja049694s
-
A General Procedure for the Esterification of Carboxylic Acids with Diazoalkanes Generated in Situ by the Oxidation of N-tert-Butyldimethylsilylhydrazones with (Difluoroiodo)Benzene.
Furrow M.; Myers A. J Am Chem Soc 2004, 126 (39), 12222–12223. DOI: 10.1021/ja0459779