CAS 93102-05-7, Cat. No EN300-54483
Precursor of non-stabilized azomethine ylide
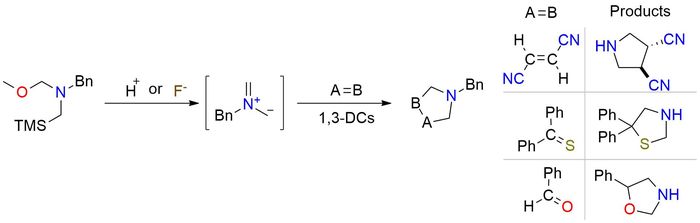
N-benzyl-N-(methoxymethyl)-N-trimethylsilylmethylamine is a convenient source of non-stabilized azomethine ylide that plays as a 1,3-dipole for many [3+2] or [3+3] cycloaddition reactions in the construction of functionalized N-heterocycles1,2,3,4. The intermediate forms in metal-free conditions in the presence of trifluoroacetic acid, zinc chloride, or cesium fluoride5. Reactive targets include classical ones - aliphatic aldehydes, aryl isothiocyanates, or activated four- and five-membered alkenes, but also medical-appropriate C,N-cyclic azomethine imines, vinyl sulfonyl fluorides, cyanosulfones, and alkenyl boropinacolates2.
Synonyms: N-benzyl-N-(methoxymethyl)-N-trimethylsilylmethylamine; N-(methoxymethyl)-N-(trimethylsilylmethyl)benzylamine; N-benzyl-1-methoxy-N-((trimethylsilyl)methyl); methenamine benzyl(methoxymethyl)[(trimethylsilyl)methyl]amine; N-(methoxymethyl)-1-phenyl-N-(trimethylsilylmethyl)methenamine
Selected publications
-
Synthesis of 6‐Azaspiro[4.3]Alkanes: Innovative Scaffolds for Drug Discovery.
Chalyk B.; Isakov A.; Butko M.; Hrebeniuk K.; Savych O.; Kucher O.; Gavrilenko K.; Druzhenko T.; Yarmolchuk V.; Zozulya S.; Mykhailiuk P. European J Org Chem 2017, 2017 (31), 4530–4542. DOI: 10.1002/ejoc.201700536
-
Bicyclic Pyrrolidines for Medicinal Chemistry via [3+2]-Cycloaddition.
Savych V.; Mykhalchuk V.; Melnychuk P.; Isakov A.; Savchuk T.; Timoshenko V.; Siry S.; Pavlenko S.; Kovalenko D.; Hryshchuk O.; Reznik V.; Chalyk B.; Yarmolchuk V.; Rusanov E.; Mykhailiuk P. J Org Chem 2021, 86 (19), 13289–13309. DOI: 10.1021/acs.joc.1c01327
-
[3+2] Cycloaddition of Alkynyl Boronates and in Situ Generated Azomethine Ylide.
Liashuk O.; Ryzhov I.; Hryshchuk O.; Volovenko Y.; Grygorenko O. Chemistry – A European Journal 2024. DOI: 10.1002/chem.202303504
-
Synthesis of 3‐Borylated Pyrrolidines by 1,3‐Dipolar Cycloaddition of Alkenyl Boronates and Azomethine Ylide.
Liashuk O.; Ryzhov I.; Hryshchuk O.; Vashchenko B.; Melnychuk P.; Volovenko Y.; Grygorenko O. Chemistry – A European Journal 2022, 28 (54). DOI: 10.1002/chem.202202117
-
N-Benzyl-N-(Methoxymethyl)-N-Trimethylsilylmethylamine.
Orlek B. Encyclopedia of Reagents for Organic Synthesis 2001. DOI: 10.1002/047084289X.rb064