CAS 157141-27-0, Cat. No EN300-316330
Reagent for the Mitsunobu reaction
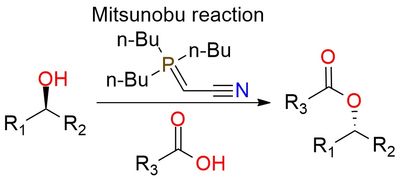
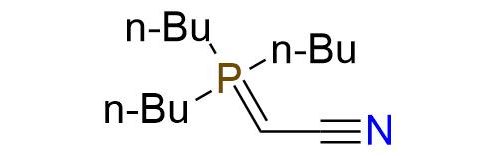
(Cyanomethylene)tributylphosphorane (CMBP or Tsunoda reagent) is a stabilized trialkylphosphorane that serves as a versatile substitute for diethyl azodicarboxylate (DEAD) and triphenylphosphine (PPh3) in the Mitsunobu reaction1. It exhibits comparable performance to PPh3 and DEAD combining advantages within a single molecule. What sets CMBP apart is its enhanced reactivity and compatibility with high temperatures, enabling the utilization of weakly acidic pronucleophiles beyond the typical pKa range for the Mitsunobu reaction2. Additionally, CMBP offers a clean reaction profile, eliminating the need for phosphine nucleophile reagents and generating acetonitrile as an innocuous by-product, leading to a simple purification process. The CMBP-mediated reaction is carried out at ambient temperature, leading to the product with excellent yield and purity, but may require more time.
Synonyms: (cyanomethylene)tributylphosphorane; CMBP; 2-(tributylphosphoranylidene)acetonitrile; (tributylphosphoranylidene)acetonitrile; 2-(tributyl-lambda5-phosphanylidene)acetonitrile
Selected publications
-
(Tributylphosphoranylidene)Acetonitrile.
Wyatt P. Encyclopedia of Reagents for Organic Synthesis 2008. DOI: 10.1002/047084289X.rn00790
-
Novel Reactivity of Stabilized Methylenetributylphosphorane: A New Mitsunobu Reagent.
Tsunoda T.; Ozaki F.; Itô S. Tetrahedron Lett 1994, 35 (28), 5081–5082. DOI: 10.1016/S0040-4039(00)73326-0