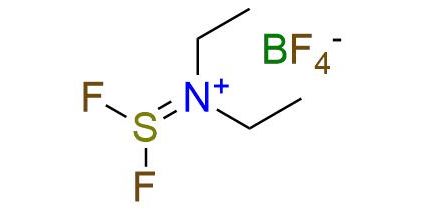
CAS 63517-29-3, Cat. No EN300-393165
Deoxofluorinating reagent and activating agent of some organic groups
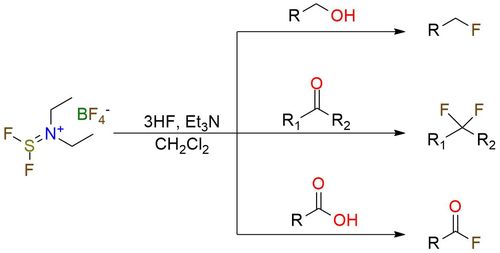
Diethylaminodifluorosulfinium tetrafluoroborate (XtalFluor-E) is a powerful and versatile fluorinating agent that surpasses traditional reagents in multiple aspects. XtalFluor-E offers several advantages over its renowned precursor, DAST1. The salt form exhibits enhanced handling ease and higher temperature stability2. As a deoxofluorinating reagent, XtalFluor-E demonstrates superior selectivity compared to SF4 and generates fewer elimination byproducts3,4. Beyond this, XtalFluor-E also serves as an activating reagent for substrates such as alcohols and carboxylic acids, promoting the substitution of oxygen by other nucleophiles like halides or amines1,4. Additionally, it facilitates regioselective aziridine ring opening, leading to the formation of fluorinated diamino acid derivatives.
Synonyms: XtalFluor-E®; (diethylamino)difluorosulfonium tetrafluoroborate; diethylamino(difluoro)sulfanium tetrafluoroborate
Selected publication
-
Diethylaminodifluorosulfinium Tetrafluoroborate (XtalFluor-E®).
Mahé O.; Paquin J.Encyclopedia of Reagents for Organic Synthesis 2013. DOI: 10.1002/047084289X.rn01631
-
Aminodifluorosulfinium Tetrafluoroborate Salts as Stable and Crystalline Deoxofluorinating Reagents.
Beaulieu F.; Beauregard L.; Courchesne G.; Couturier M.; LaFlamme F.; L’Heureux A. Org Lett 2009, 11 (21), 5050–5053. DOI: 10.1021/ol902039q
-
Synthesis and Chemical Transformations of Diazolyl α,α-Difluoroacetates.
Geraschenko O.; Solomin V.; Vashchenko B.; Khodakivskyi P.; Tolmachev A.; Grygorenko O. J Fluor Chem 2020, 229, 109407. DOI: 10.1016/j.jfluchem.2019.109407
-
A Novel and Selective Fluoride Opening of Aziridines by XtalFluor-E. Synthesis of Fluorinated Diamino Acid Derivatives.
Nonn M.; Kiss L.; Haukka M.; Fustero S.; Fülöp F. Org Lett 2015, 17 (5), 1074–1077. DOI: 10.1021/acs.orglett.5b00182