CAS 1416881-52-1, Cat. No EN300-6487157
Catalyst for photoredox C-C cross-coupling
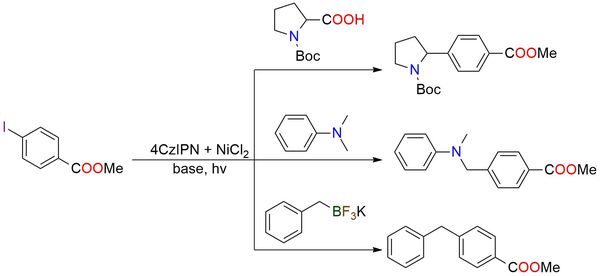
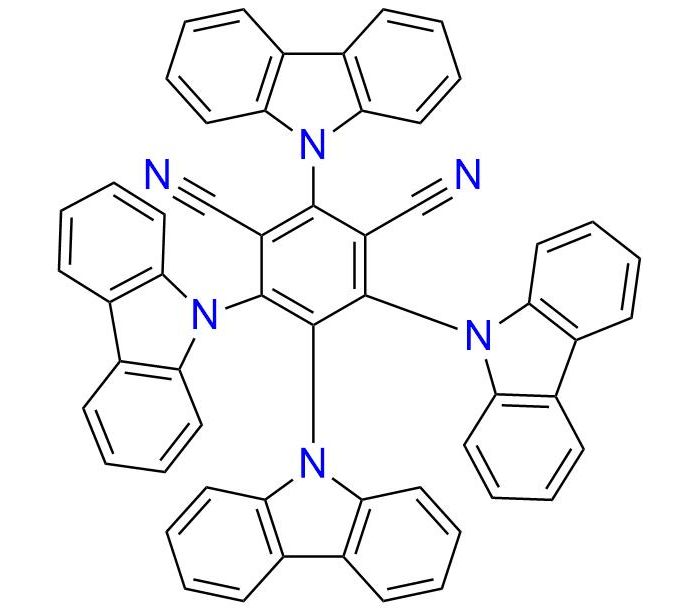
2,4,5,6-tetra(9H-Carbazol-9-yl)isophthalonitrile (4CzIPN) is a donor-acceptor chromophore and efficient metal-free photoredox catalyst for organic synthesis1. The photocatalyst is a powder, that is stable in the fridge for an extended period and easy to handle2. It is a well-known catalyst in photoredox/Ni dual catalytic C(sp3)−C(sp2) cross-coupling where 4CzIPN demonstrates exceptional efficacy in arylation of a wide scope of substrates3,4. It enables amines alpha-arylation, α-amino acids decarboxylation-arylation, and trifluoroborates arylation with good yields, utilizing only a catalytic amount of the 4CzIPN3–5. The process can be performed under soft conditions without strong UV irradiation and with blue LED light1,5.
Synonyms: 4CzIPN; 2,4,5,6-tetra(carbazol-9-yl)isophthalonitrile; 2,4,5,6-tetra(carbazol-9-yl)benzene-1,3-dicarbonitrile; tetrakis(9H-carbazol-9-yl)benzene-1,3-dicarbonitrile; 1,3-benzenedicarbonitrile, 2,4,5,6-tetra-9H-carbazol-9-yl-
Selected publication
-
Organic Photoredox Catalysis.
Romero N.; Nicewicz D. Chem Rev 2016, 116 (17), 10075–10166. DOI: 10.1021/acs.chemrev.6b00057
-
1,3-Benzenedicarbonitrile, 2,4,5,6-Tetra-9H-Carbazol-9-yl-.
Lu J.; Gui R.; Zhang J. Encyclopedia of Reagents for Organic Synthesis 2017, 1–2. DOI: 10.1002/047084289X.rn02068
-
Single-Electron Transmetalation in Organoboron Cross-Coupling by Photoredox/Nickel Dual Catalysis.
Tellis J.; Primer D.; Molander G. Science (1979) 2014, 345 (6195), 433–436. DOI: 10.1126/science.1253647
-
Merging Photoredox with Nickel Catalysis: Coupling of α-Carboxyl sp3-Carbons with Aryl Halides.
Zuo Z.; Ahneman D.; Chu L.; Terrett J.; Doyle A.; MacMillan D. Science (1979) 2014, 345 (6195), 437–440. DOI: 10.1126/science.1255525
-
Donor–Acceptor Fluorophores for Visible-Light-Promoted Organic Synthesis: Photoredox/Ni Dual Catalytic C(sp3)–C(sp2) Cross-Coupling.
Luo J.; Zhang J. ACS Catal 2016, 6 (2), 873–877. DOI: 10.1021/acscatal.5b02204