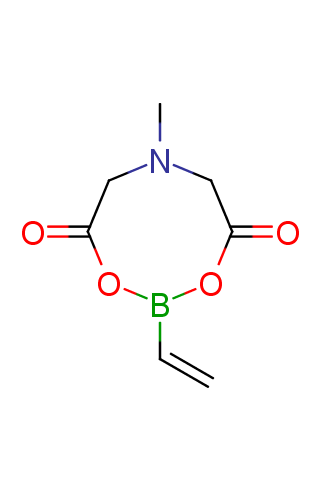
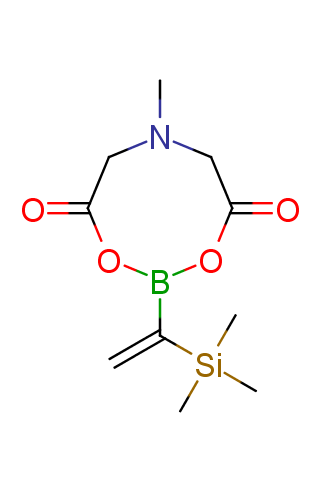
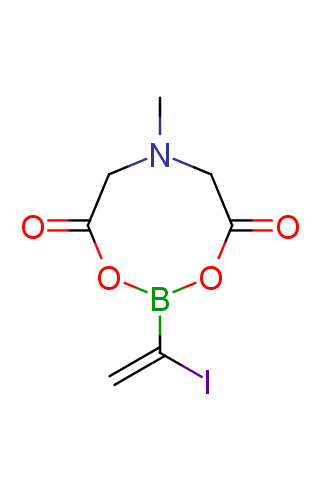
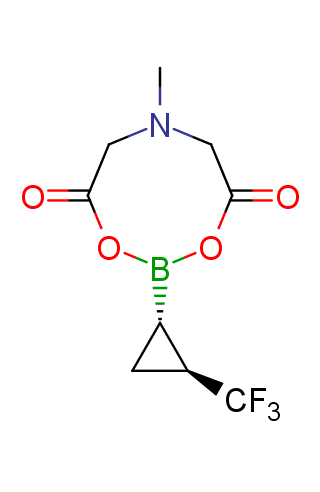
The transition-metal-catalyzed cross-coupling reactions are powerful tools for the formation of carbon–carbon bonds. Among these transformations, the Suzuki–Miyaura reaction, which is the transition-metal-catalyzed cross-coupling between an organo-boron compound and an organic (pseudo)halide, has become the most attractive method since its discovery in 1979. While many organo-boron compounds have been discovered, N-methyliminodiacetic acid MIDA boronates represent tremendously useful building blocks for the Suzuki–Miyaura cross-coupling that has been successfully applied to sequential synthesis of various natural product motifs.
Features of MIDA boronates:
- Stable to benchtop storage under air;
- Compatible with common functional groups (FG);
- Compatible with wide range of common synthetic reagents;
- Tolerate harsh reaction conditions (strongly acidic, oxidative, etc.);
- Easily hydrolyzed using mild conditions;
- Compatible with chromatography.
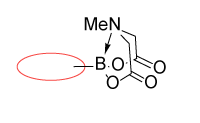
Terminal MIDA boronates
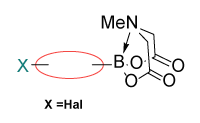
Linker MIDA boronates
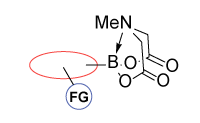
Functionalized MIDA boronates
Enamine offers a collection of (hetero)aryl and alkyl MIDA boronates including terminal, linker, and functionalized building blocks that allows modifying or sequential assembling molecules of interest.