Tetrahedron: Asymmetry , 2014, 25 (3), 229‑237
DOI: 10.1016/j.tetasy.2013.12.001
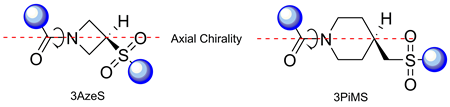
The conformational behavior of four model peptides containing residues of 3-azetidinesulfonic (3AzeS) and 4-piperidinemethane sulfonic (4PiMS) acids was studied in both a crystalline state and in solution using X-ray, NMR, and IR experiments. It was found that in the crystalline state, both of the models di- and tripeptides studied adopted extended conformations and demonstrated considerable conformational flexibility. In solution, it is likely that a flexible ensemble of conformations is adopted, including extended structures and more compact ones without persistent hydrogen bonds. One of the most interesting features of the peptides was the axial chirality observed due to the slow rotation around the amide bond formed by the endocyclic nitrogen atoms of the non-chiral 3AzeS and 4PiMS residues. It was shown for one of the derivatives that the configuration of the chiral axis had an impact on the conformation of the neighboring amino acid residue.
Grygorenko O. O.; Zhersh S.; Oliinyk B. V.; Shishkin O. V.; Tolmachev A. A.
Tetrahedron: Asymmetry 2014, 25 (3), 229-237
DOI: 10.1016/j.tetasy.2013.12.001
