J. Heterocycl. Chem. 2018, 55 (10), 2381-2391
DOI: 10.1002/jhet.3302
Tkachenko I.; Tarabara I.; Omelchenko I.; Palchykov V.
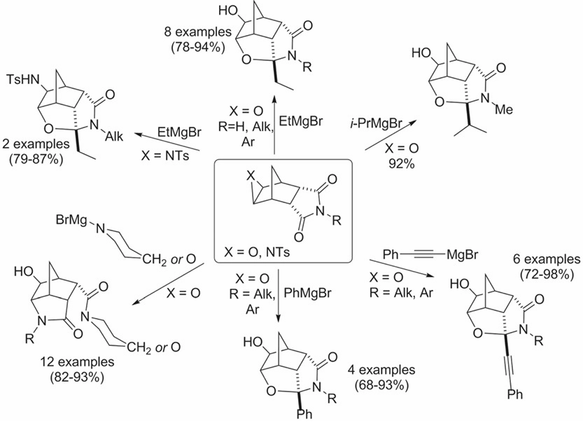
A convenient method is reported for the synthesis of 34 novel tricyclic and tetracyclic cage‐like lactams in high to excellent yields. The method involves treatment of easily available exo‐2,3‐epoxy(or Ts‐aziridino)bicyclo[2.2.1]heptan‐endo‐5,6‐dicarboximides by Grignard reagents, their N‐analogues, or phenyl lithium in tetrahydrofuran. In silico screening of biological activity spectrum showed high probability levels of glyceryl‐ether monooxygenase inhibitory, testosterone 17β‐dehydrogenase (NADP+) inhibitory, bilirubin oxidase inhibitory, and anti‐ischemic activity for tetracyclic derivatives.