RSC Adv. 2016, 6 (27), 22737-22748
DOI: 10.1039/C6RA01548D
Tymtsunik A. V.; Kokhan S. O.; Ivon Y. M.; Komarov I. V.; Grygorenko O. O.
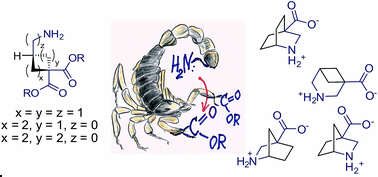
Differentiation of identical electrophilic functional groups (carboxylates) by a strategically placed internal nucleophile (an amino group) in cyclic precursors was used as a key general approach to functionalized azabicyclic scaffolds. The utility of the method was demonstrated by the synthesis of three bicyclic β-amino acids (analogues of nipecotic acid), which were prepared in good yields and on a relatively large scale.