Tetrahedron 2016, 72 (10), 1351-1356
DOI: 10.1016/j.tet.2016.01.032
Michurin O. M.; Radchenko D. S.; Komarov I. V.
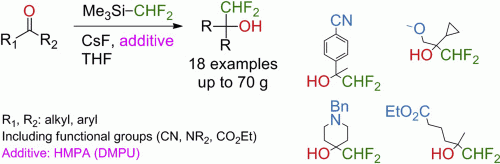
Easily available difluoromethylating reagent Me3SiCF2H enables multigram synthesis of difluoromethyl alcohols in good yields under mild conditions from a number of aldehydes and ketones in the presence of HMPA. This additive makes possible the previously challenging nucleophilic difluoromethylation of enolizable ketones. DMPU can be used as a non-toxic alternative to the HMPA in the difluoromethylation reaction, albeit the yields were slightly lower in this case. The method works well with cyclic, acyclic, aryl ketones and tolerates various functional groups.