J. Am. Chem. Soc. 2015, 137 (2), 926-930
DOI: 10.1021/ja511460a
Komarov I. V.; Yanik S.; Ishchenko A. Y.; Davies J. E.; Goodman J. M.; Kirby A. J.
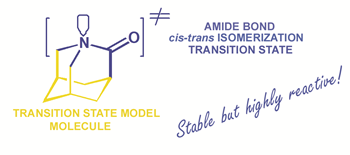
1-Azatricyclo[3.3.1.13,7]decan-2-one (3), the parent compound of a rare class of 90°-twisted amides, has finally been synthesized, using an unprecedented transformation. These compounds are of special interest as transition-state mimics for the enzyme-catalyzed cis-trans rotamer interconversion of amides involved in peptide and protein folding and function. The stabilization of the amide group in its high energy, perpendicular conformation common to both systems is shown for the rigid tricyclic system to depend, as predicted by calculation, on its methyl group substitution pattern, making 3 by some way the most reactive known "amide".