Tetrahedron Lett. 2014, 55 (22), 3312-3315
DOI: 10.1016/j.tetlet.2014.04.038
Tymtsunik A. V.; Ivon Y. M.; Komarov I. V.; Grygorenko O. O.
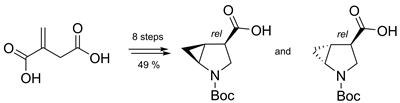
An efficient method for the preparation of Boc-protected 4,5-methano-β-proline—a novel bicyclic cyclopropane-containing β-amino acid—was developed, starting from readily available itaconic acid. A modified Simmons–Smith reaction was used for the construction of the cyclopropane ring. The method allowed for the synthesis of both cis and trans isomers of the title compound in 49% total yield and can be employed for gram-scale preparations. An approach to the preparation of methyl 5-oxopyrrolidine-3-carboxylate, which is one of the key intermediates in the synthetic scheme, on a multigram scale was also developed.