Tetrahedron , 2013, 69 (3), 1217-1228
DOI: 10.1016/j.tet.2012.11.026
Iaroshenko V. O.; Vilches-Herrera M.; Gevorgyan A.; Mkrtchyan S.; Arakelyan K.; Ostrovskyi D.; Abbasi M. S. A.; Supe L.; Hakobyan A.; Villinger A.; Volochnyuk D. M.; Tolmachev A.
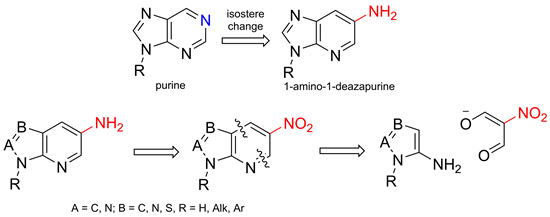
The synthesis of 1-deazapurines and isosteres bearing the exocyclic nitrogen atom at position-1 was developed basing on the formal [3+3]-cyclization reaction of nitro-malonaldehyde with the set of electron-excessive aminoheterocycles. Through the functionalization of the purine-like scaffolds synthesized the diversity of compounds furnished in the possition-1 with aryl, alkinyl, and vinyl rests, were obtained.
Iaroshenko V. O.; Vilches-Herrera M.; Gevorgyan A.; Mkrtchyan S.; Arakelyan K.; Ostrovskyi D.; Abbasi M. S. A.; Supe L.; Hakobyan A.; Villinger A.; Volochnyuk D. M.; Tolmachev A.
Tetrahedron 2013, 69 (3), 1217-1228
DOI: 10.1016/j.tet.2012.11.026
