Tetrahedron , 2011, 67 (34), 6233-6239
DOI: 10.1016/j.tet.2011.06.063
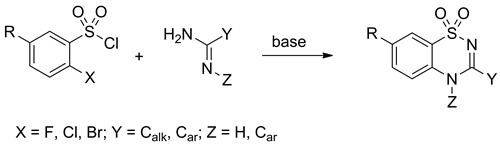
Condensations of o-halo-substituted benzenesulfonyl chlorides with 2-aminopyridines and amidines may give the corresponding 1,2,4-benzothiadiazine-1,1-dioxides under mild, non-catalytic conditions in nearly quantitative yields. The successful one-pot cyclization depends on three factors: (i) the nature of the o-halogen, (ii) the electronic character of the benzene ring substituent, and (iii) the steric load around the amidine unit. O-Fluorobenzenesulfonyl chlorides bearing methylcarboxyl- or nitro-group and o-chloro- and o-bromobenzenesulfonyl chlorides bearing nitro-group are reactive enough to give the desired 1,2,4-benzothiadiazine-1,1-dioxides in a one-pot base-promoted reaction. In all other cases, open-chain sulfonylated amidine intermediates are isolated. The latter are converted to the title compounds either in the presence of potassium carbonate or upon the addition of a copper(I) catalyst.
Cherepakha A.; Kovtunenko V. O.; Tolmachev A.; Lukin O.
Tetrahedron 2011, 67 (34), 6233-6239
DOI: 10.1016/j.tet.2011.06.063
