Synthesis , 2010, 11, 1781-1792
DOI: 10.1055/s-0029-1219760
Maximov N. B.; Mykhailiuk P. K.; Golovach S. M.; Tverdokhlebov A. V.; Voitenko Z. V.; Tolmachev A. A.
The reaction of ethyl 5-acetyl-3,4-dihydropyridine-1(2H)-carboxylate with diverse aliphatic as well as aromatic monosubstituted hydrazines resulted in the regioselective formation of N-substituted 3-methylpyrazoles.
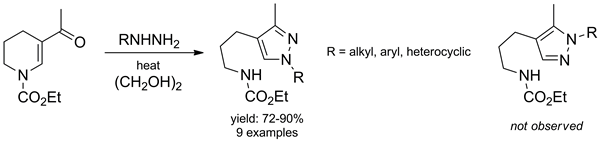
Maximov N. B.; Mykhailiuk P. K.; Golovach S. M.; Tverdokhlebov A. V.; Voitenko Z. V.; Tolmachev A. A.
Synthesis 2010, 11, 1781-1792
DOI: 10.1055/s-0029-1219760
