Synthesis , 2010, 16, 2749-2758
DOI: 10.1055/s-0029-1218842
Iaroshenko V. O.; Mkrtchyan S.; Volochnyuk D. M.; Langer P.; Sosnovskikh V. Y.; Ostrovskyi D.; Dudkin S.; Kotljarov A. V.; Miliutina M.; Savych I.; Tolmachev A. A.
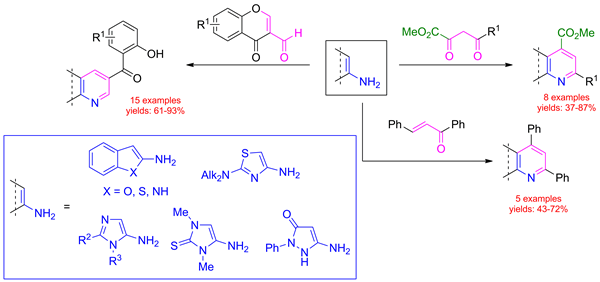
The reaction of electron-rich aminoheterocycles with 1,3-CCC-dielectrophiles, such as 3-formylchromones, acylpyruvates, and chalcone, provided diversely fused pyridines. Starting from 5-amino-1-(2,3-O-isopropylidene-β-d-ribofuranosyl)-1H-pyrazole, nucleosides containing a pyrazolo[3,4-b]pyridine fragment were obtained, which can be considered as adenosine deaminase (ADA) inhibitors.
Iaroshenko V. O.; Mkrtchyan S.; Volochnyuk D. M.; Langer P.; Sosnovskikh V. Y.; Ostrovskyi D.; Dudkin S.; Kotljarov A. V.; Miliutina M.; Savych I.; Tolmachev A. A.
Synthesis 2010, 16, 2749-2758
DOI: 10.1055/s-0029-1218842
