J. Org. Chem. , 2009, 74 (15), 5541-5544
DOI: 10.1021/jo900842w
Radchenko D. S.; Kopylova N.; Grygorenko O. O.; Komarov I. V.
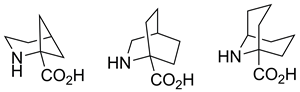
Practical syntheses of 2-azabicyclo[3.1.1]heptane-1-carboxylic (2,4-methanopipecolic), 2-azabicyclo[2.2.2]octane-1-carboxylic (2,5-ethanopipecolic), and 9-azabicyclo[3.3.1]nonane-1-carboxylic (2,6-propanopipecolic) acids are reported. The synthetic schemes are short (five, seven, and five steps, respectively) and result in reasonably high yields of the title compounds. The key step in the syntheses is the tandem Strecker reaction and intramolecular nucleophilic cyclization of ketones possessing a leaving group at the δ-position.
Radchenko D. S.; Kopylova N.; Grygorenko O. O.; Komarov I. V.
J. Org. Chem. 2009, 74 (15), 5541-5544
DOI: 10.1021/jo900842w
