Synthesis , 2007, 03, 417-427
DOI: 10.1055/s-2007-965881
Ryabukhin S. V.; Plaskon A. S.; Ostapchuk E. N.; Volochnyuk D. M.; Tolmachev A. A.
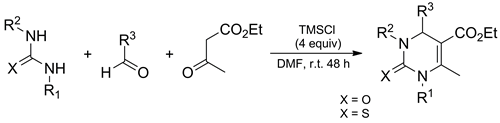
The classical Biginelli reaction has been extended by the use of N-substituted ureas and thioureas. A set of N1-alkyl-, N1-aryl-, and N1,N3-dialkyl-3,4-dihydropyrimidin-2(1H)-(thi)ones was readily prepared in excellent yield when chlorotrimethylsilane in N,N-dimethylformamide was used as promoter and water scavenger.
Ryabukhin S. V.; Plaskon A. S.; Ostapchuk E. N.; Volochnyuk D. M.; Tolmachev A. A.
Synthesis 2007, 03, 417-427
DOI: 10.1055/s-2007-965881
