Org. Lett. , 2007, 9 (21), 4215-4218
DOI: 10.1021/ol701782v
Ryabukhin S. V.; Plaskon A. S.; Ostapchuk E. N.; Volochnyuk D. M.; Shishkin O. V.; Shivanyuk A. N.; Tolmachev A. A.
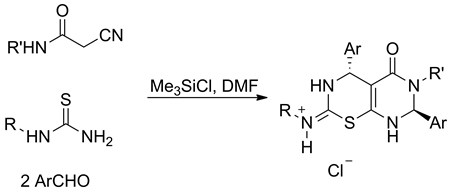
The chlorotrimethylsilane-promoted Biginelli type reactions of aldehydes, thiourea, and cyanoketones led to a diverse set of tetrahydropyrimidine-2(1H)-thiones. Under similar conditions, thioureas, benzaldehyde, and cyanoacetamide reacted to give first representatives of hexahydro-5H-pyrimido[5,4-e][1,3]thiazin-5-ones in high preparative yield.
Ryabukhin S. V.; Plaskon A. S.; Ostapchuk E. N.; Volochnyuk D. M.; Shishkin O. V.; Shivanyuk A. N.; Tolmachev A. A.
Org. Lett. 2007, 9 (21), 4215-4218
DOI: 10.1021/ol701782v
