Synthesis , 2007, 24, 3891-3895
DOI: 10.1055/s-2007-990869
Degtyarenko A. S.; Tolmachev A. A.; Volovenko Y. M.; Tverdokhlebov A. V.
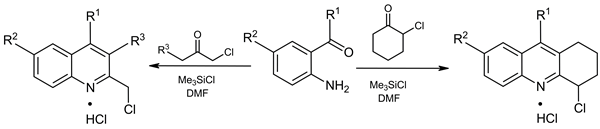
Condensation of 2-aminoacetophenone and 2-aminobenzophenone with ethyl 4-chloro-3-oxobutanoate in the presence of excess chlorotrimethylsilane was shown to give ethyl 2-chloromethyl-3-quinoline carboxylates in high yields. A similar reaction with 1,3-dichloroacetone and 2-chlorocyclohexanone led to the 3-chloro-2-(chloromethyl)quinolines and 4-chloro-1,2,3,4-tetrahydroacridines, respectively.
Degtyarenko A. S.; Tolmachev A. A.; Volovenko Y. M.; Tverdokhlebov A. V.
Synthesis 2007, 24, 3891-3895
DOI: 10.1055/s-2007-990869
