Tetrahedron: Asymmetry , 2006, 17 (2), 252-258
DOI: 10.1016/j.tetasy.2005.12.009
Grygorenko O. O.; Artamonov O. S.; Palamarchuk G. V.; Zubatyuk R. I.; Shishkin O. V.; Komarov I. V.
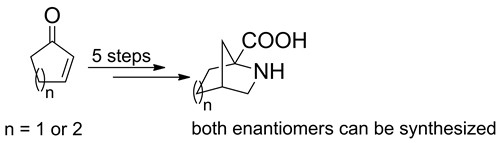
The stereoselective synthesis of 2-azabicyclo[2.2.1]heptane-1-carboxylic acid and 6-azabicyclo[3.2.1]octane-5-carboxylic acid, novel rigid bicyclic proline analogues, is reported. The synthesis was performed in five steps from the corresponding 2-cycloalken-1-ones. A known approach to 2,4-methanoproline is improved. The three amino acids constitute a library of conformationally constrained proline analogues, which can be used for the design of peptidomimetics and peptide models.
Grygorenko O. O.; Artamonov O. S.; Palamarchuk G. V.; Zubatyuk R. I.; Shishkin O. V.; Komarov I. V.
Tetrahedron: Asymmetry 2006, 17 (2), 252-258
DOI: 10.1016/j.tetasy.2005.12.009
