Chem. Heterocycl. Compd. 2020, 56 (2), 213–218
DOI: 10.1007/s10593-020-02646-z
Omelian T.; Ostapchuk E.; Dobrydnev A.; Malets Y.; Brovarets V.; Grygorenko O.
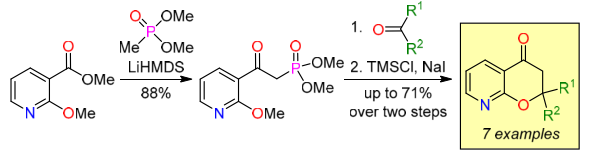
A series of 2,2-disubstituted 8-azachromanones, including spirocyclic compounds, have been synthesized via Horner–Wadsworth– Emmons reaction. Dimethyl methylphosphonate was acylated with methyl 2-methoxypyridine-3-carboxylate to afford the key intermediate – dimethyl [2-(2-methoxypyridin-3-yl)-2-oxoethyl]phosphonate. Further reaction of this phosphonate and ketones followed by treatment with TMSCl–NaI provided the target 8-azachromanones. Scope and limitations of the developed synthetic method have been investigated.