Org. Biomol. Chem. 2019, 17, 4342-4349
DOI: 10.1039/c9ob00393b
Hutskalova V.; Mykhailiuk P.
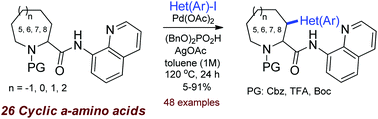
A systematic study on the directed Pd-catalyzed (hetero)arylation of 26 substituted cyclic α-amino acids at the C(3)-atom was performed. For the first time, the 7- and 8-membered cyclic amino acids were introduced to C-H activation. 8-Aminoquinoline was used as a directing group. Effects of the ring size and the substituents on the reaction efficacy and stereoselectivity were studied.