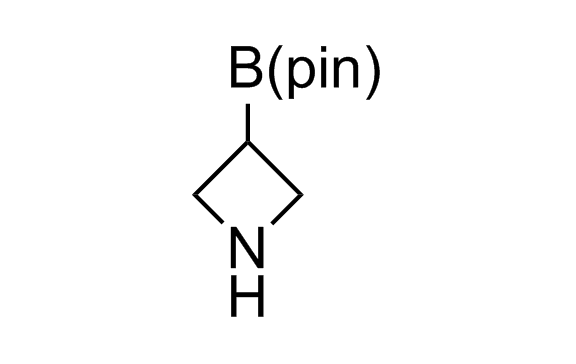
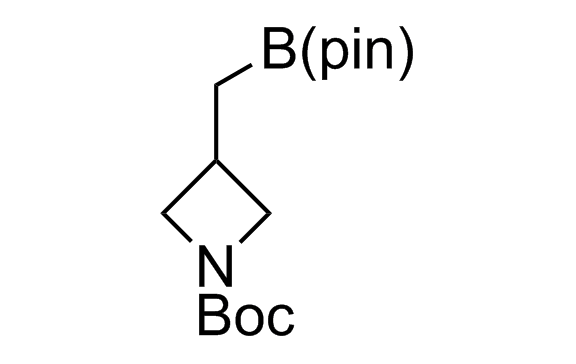
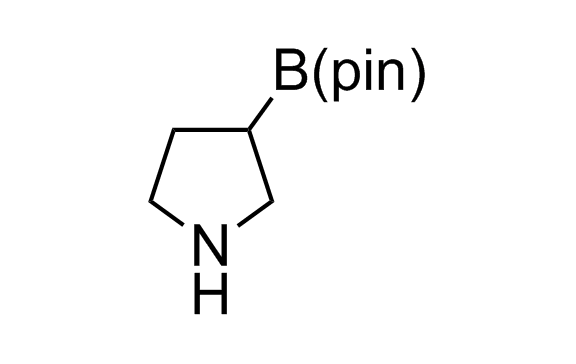
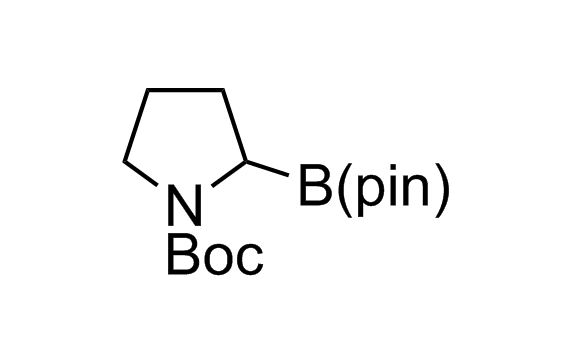
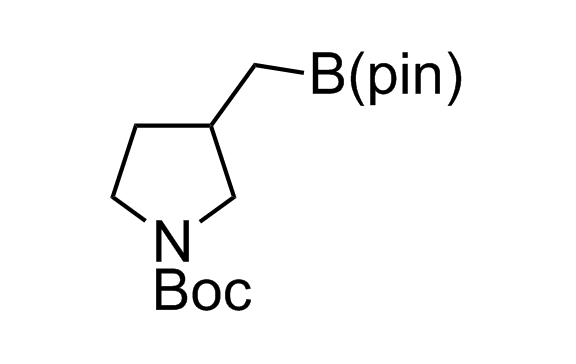
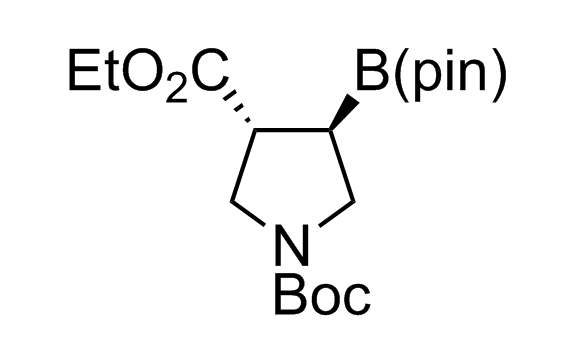
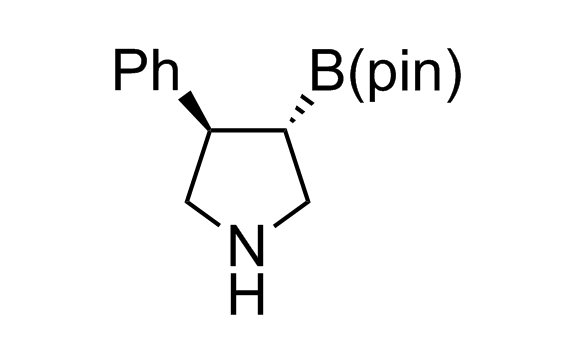
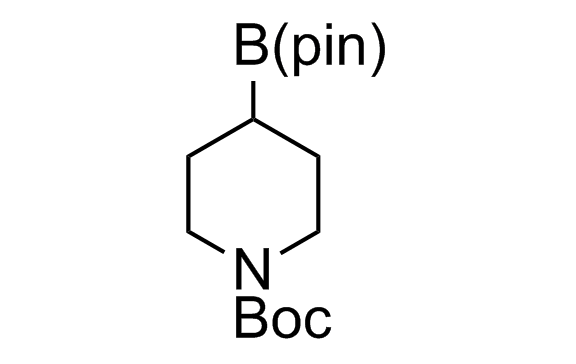
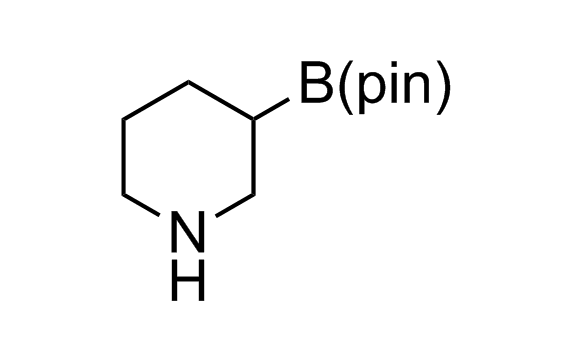
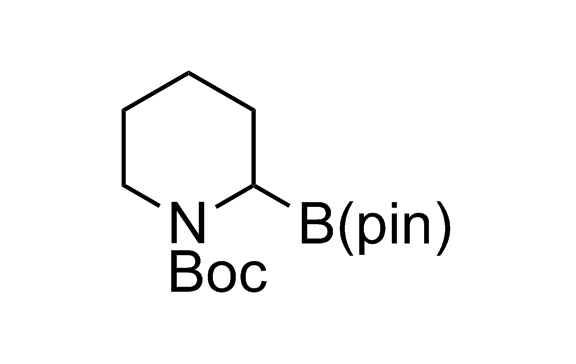
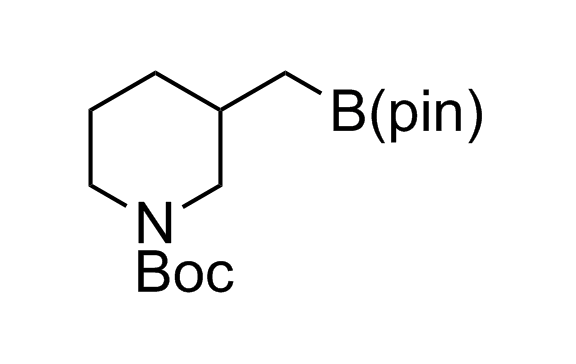
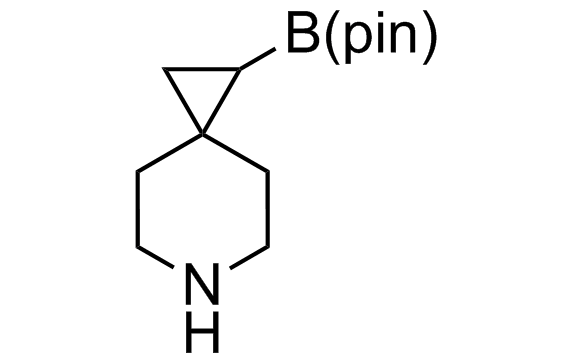
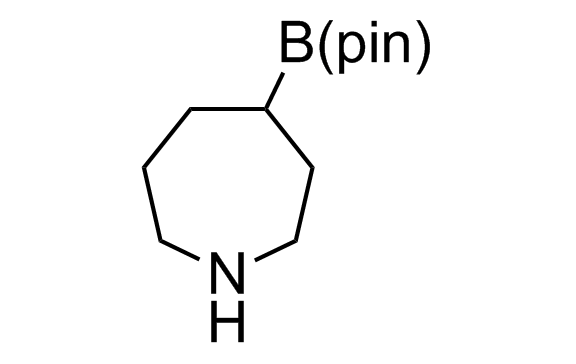
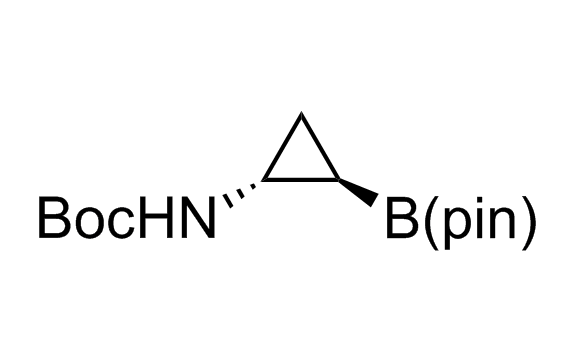
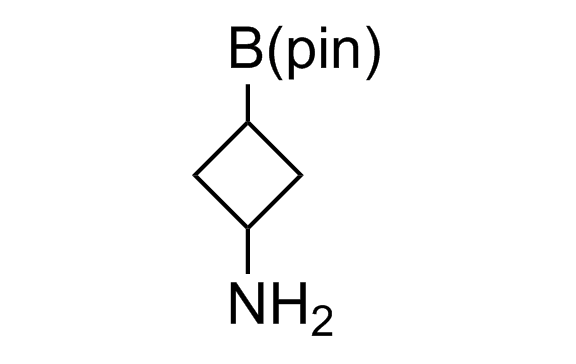
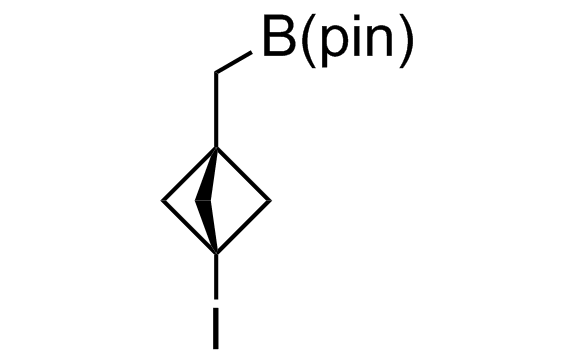
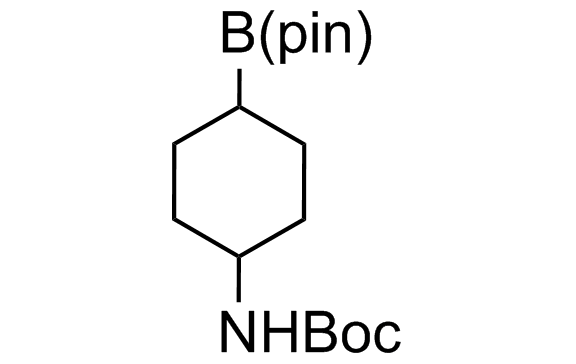
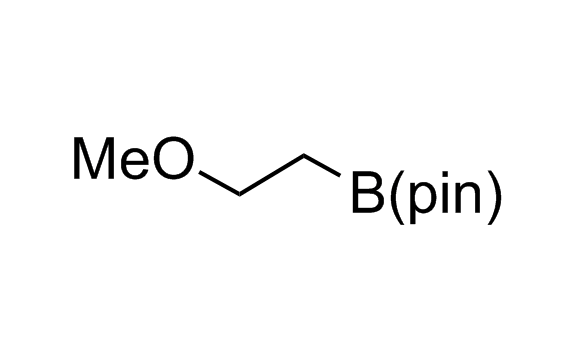
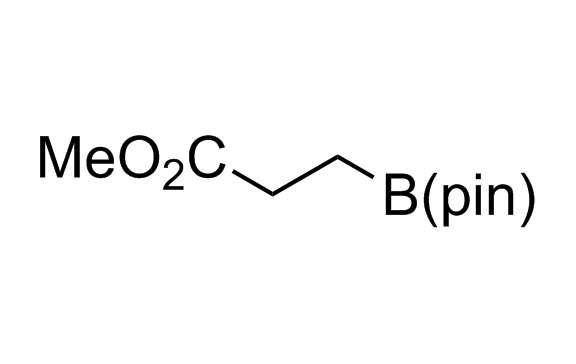
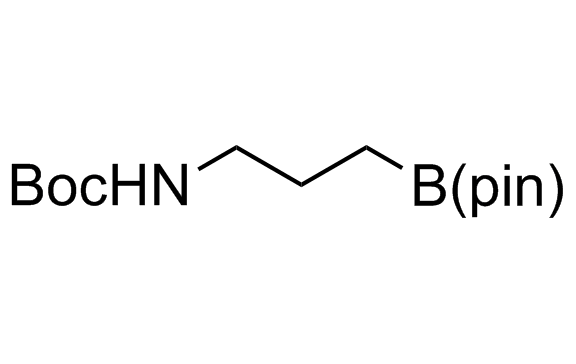
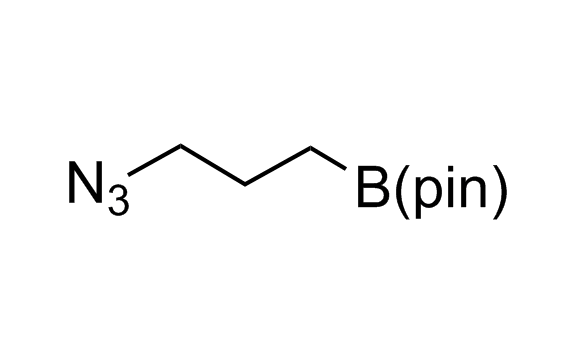
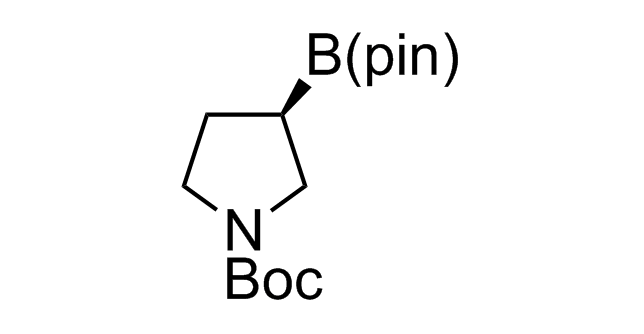
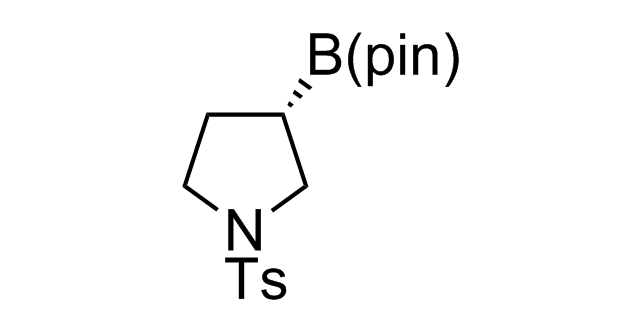
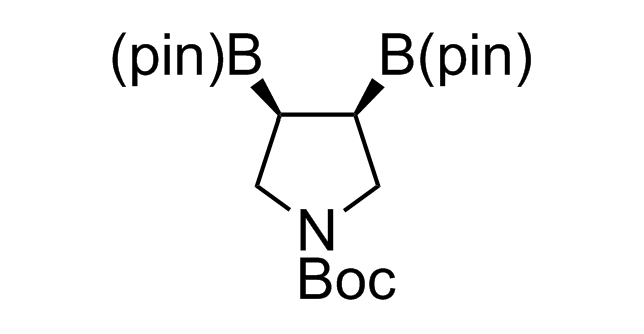
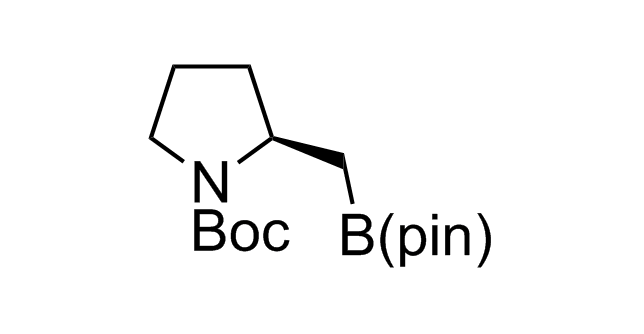
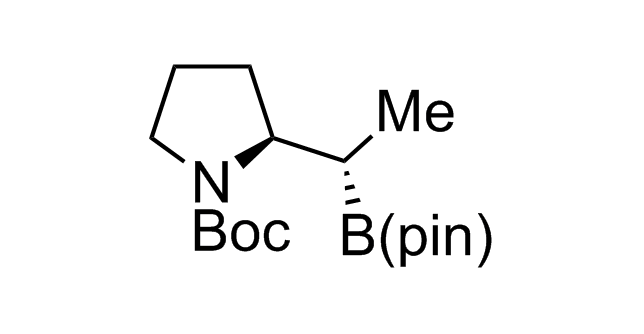
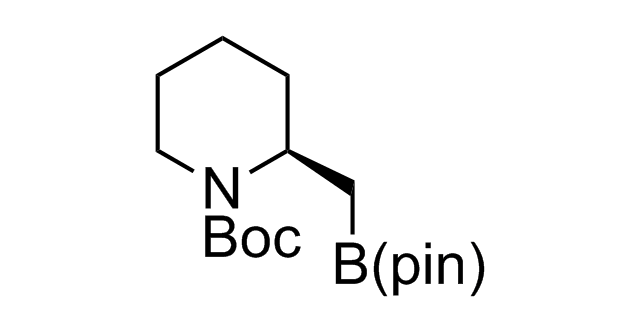
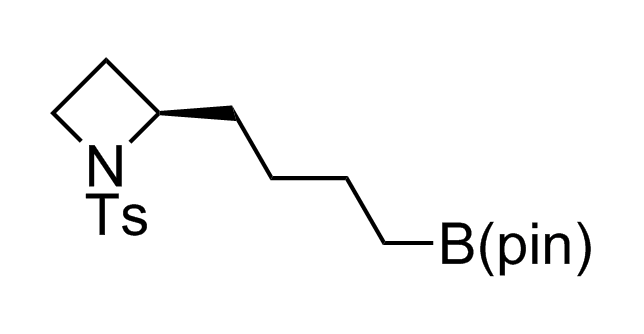
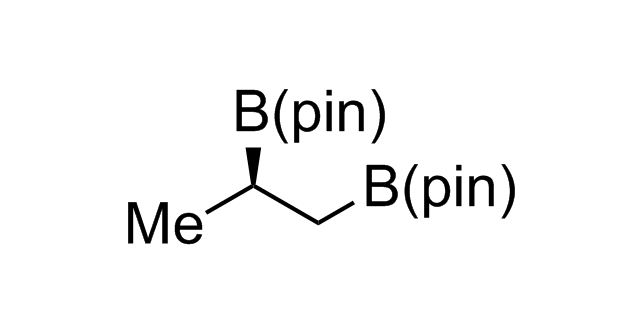
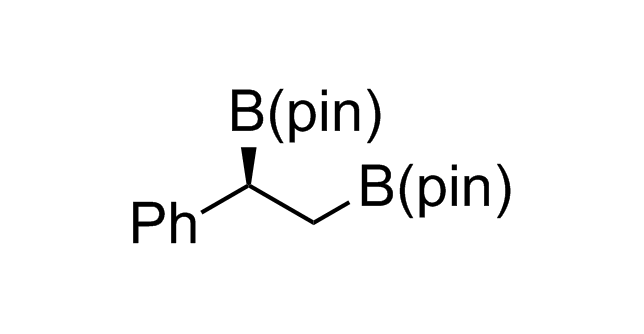
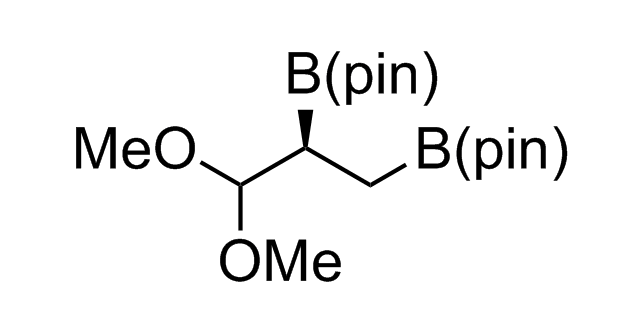
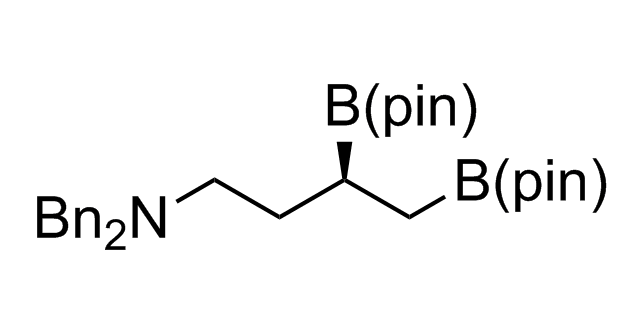
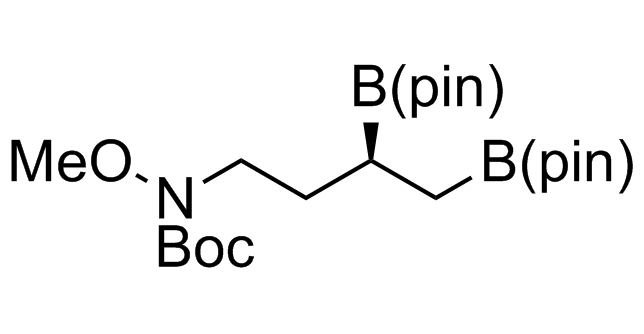
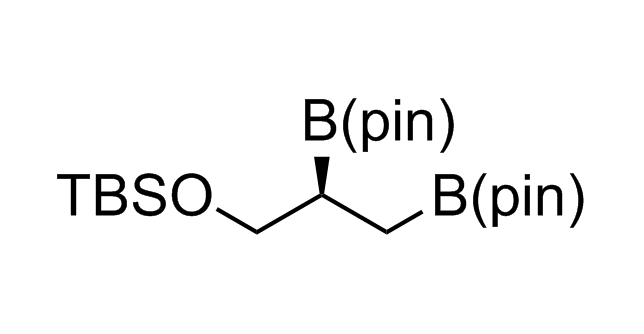
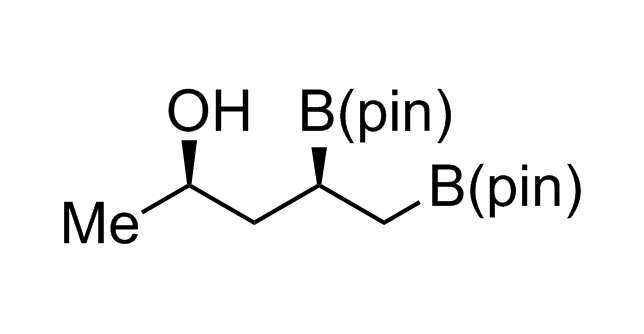
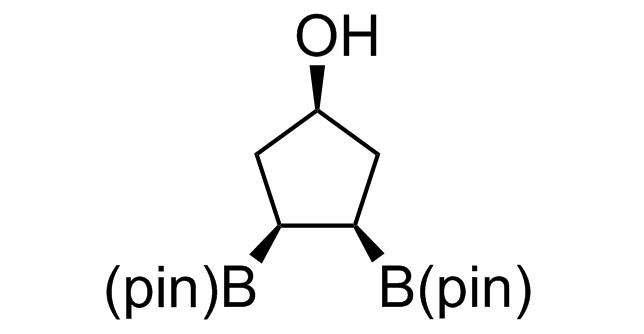
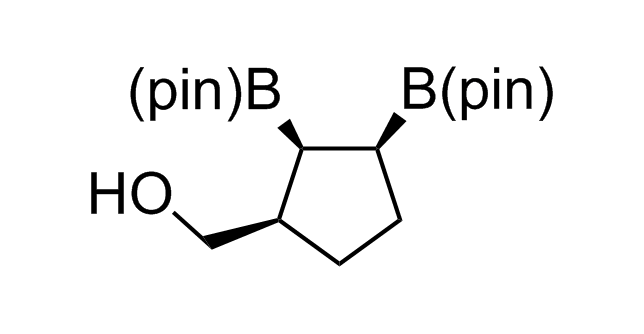
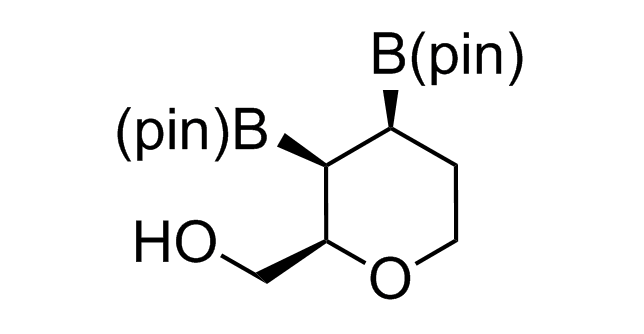
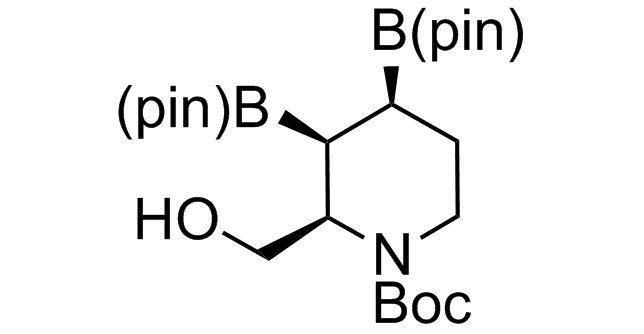
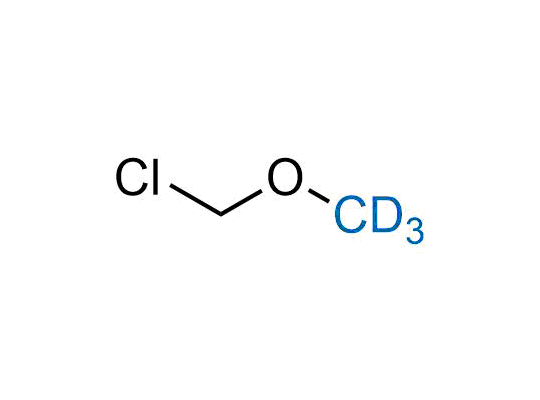
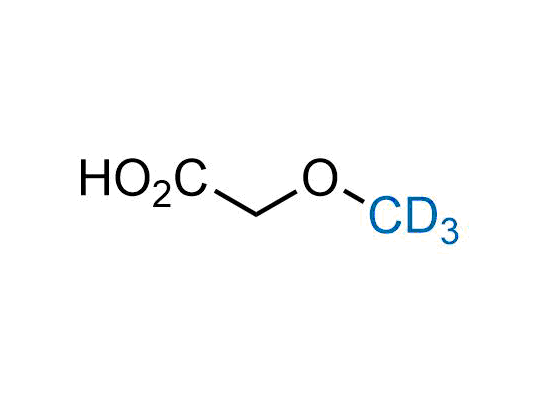
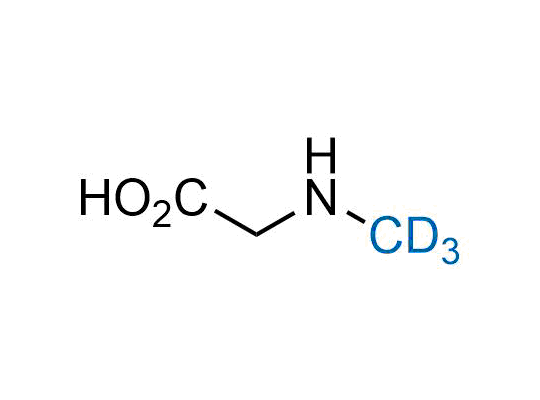
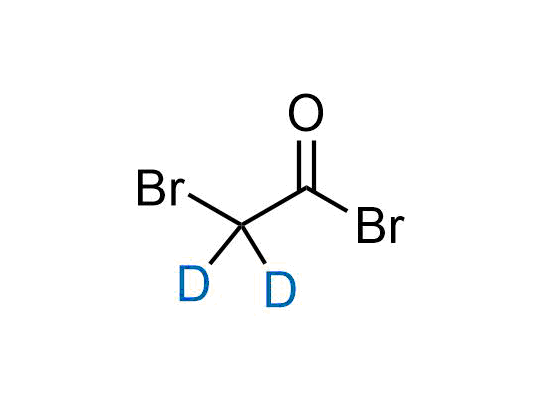
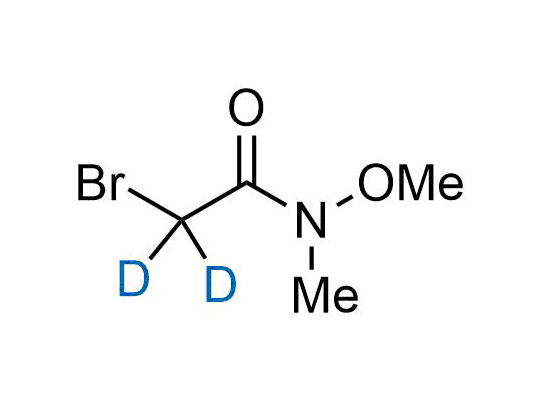
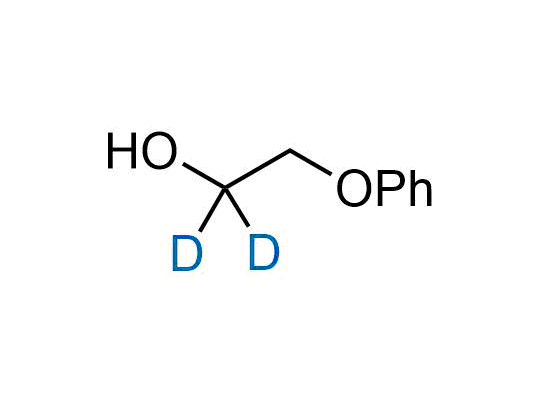
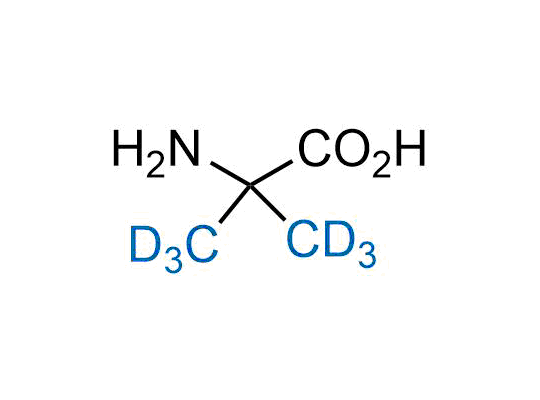
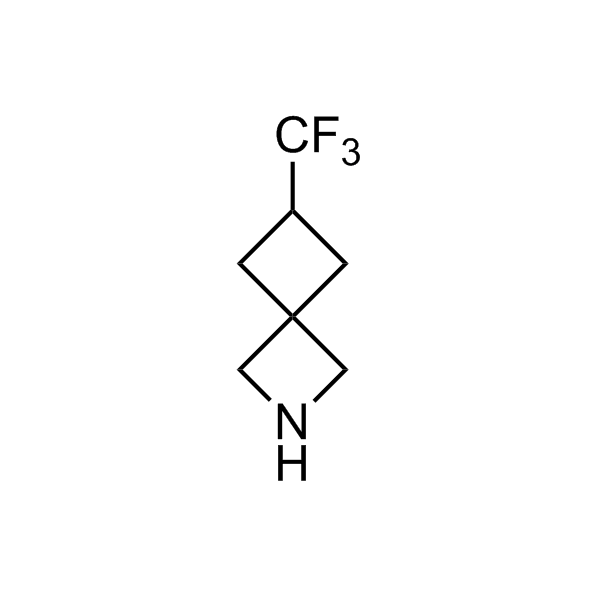
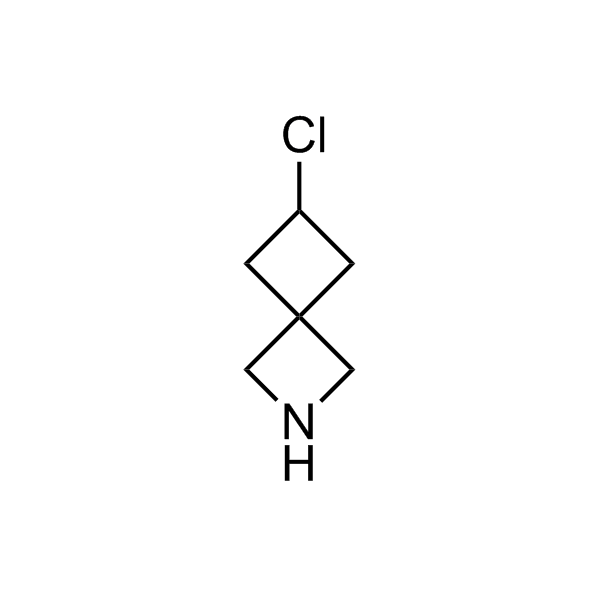
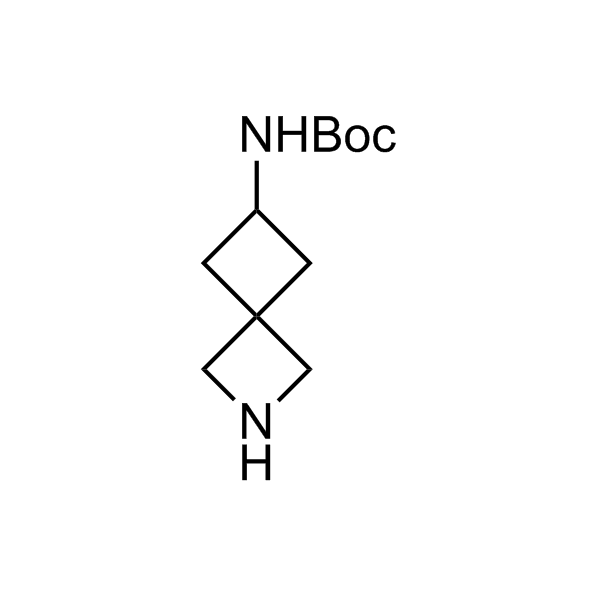
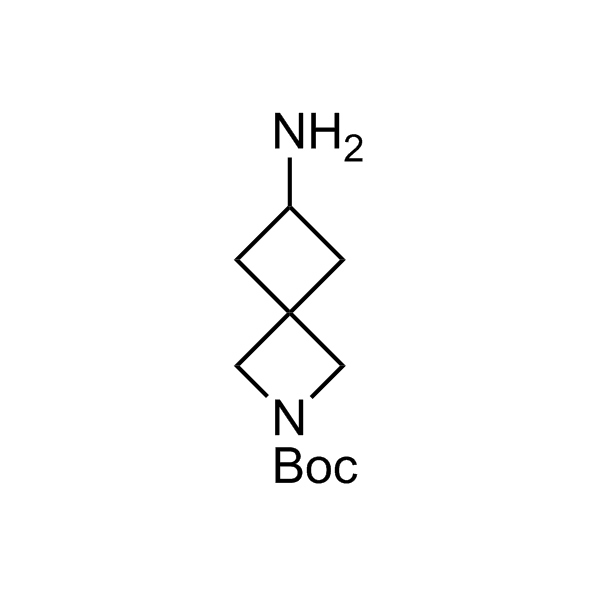
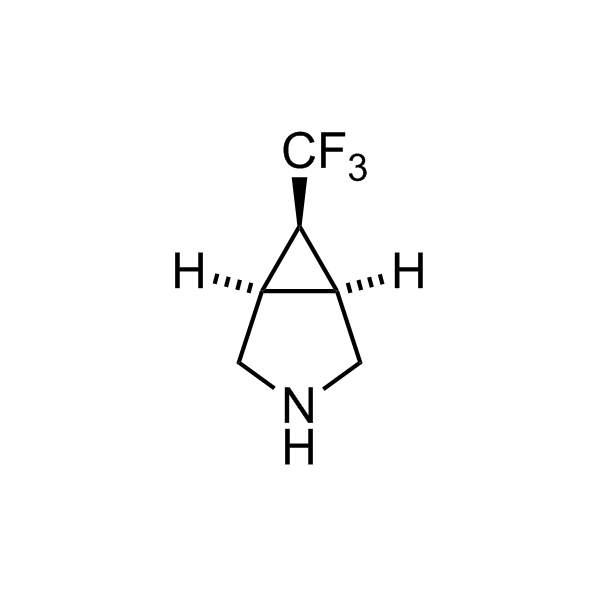
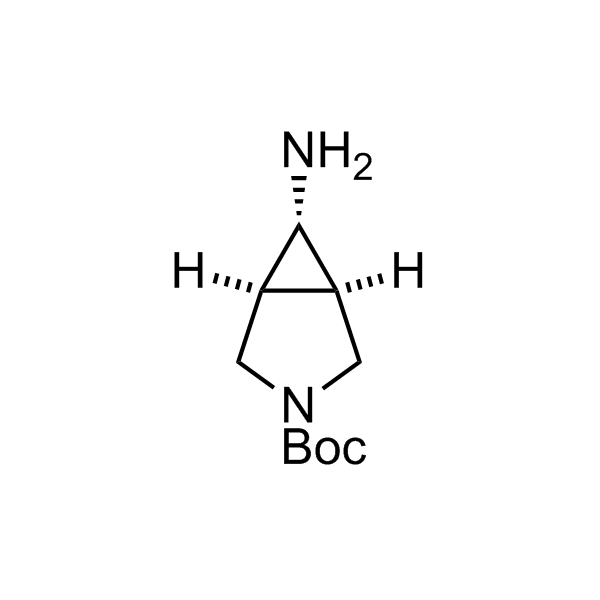
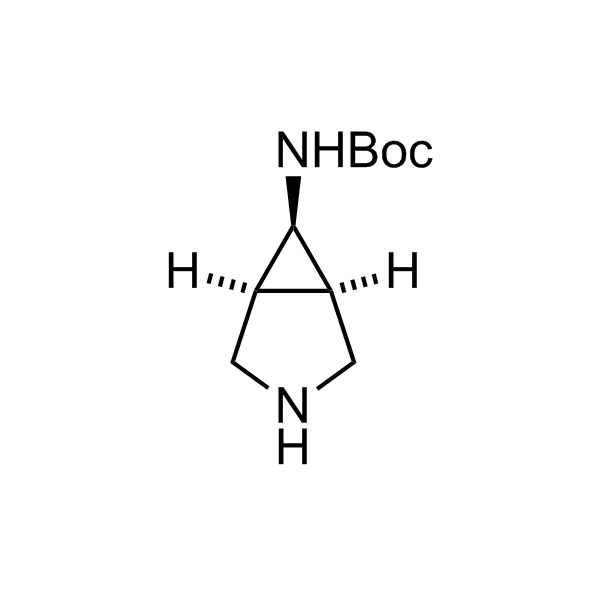
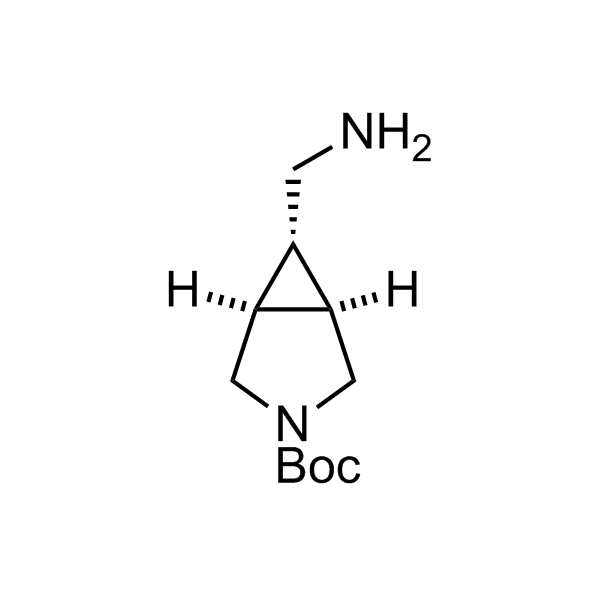
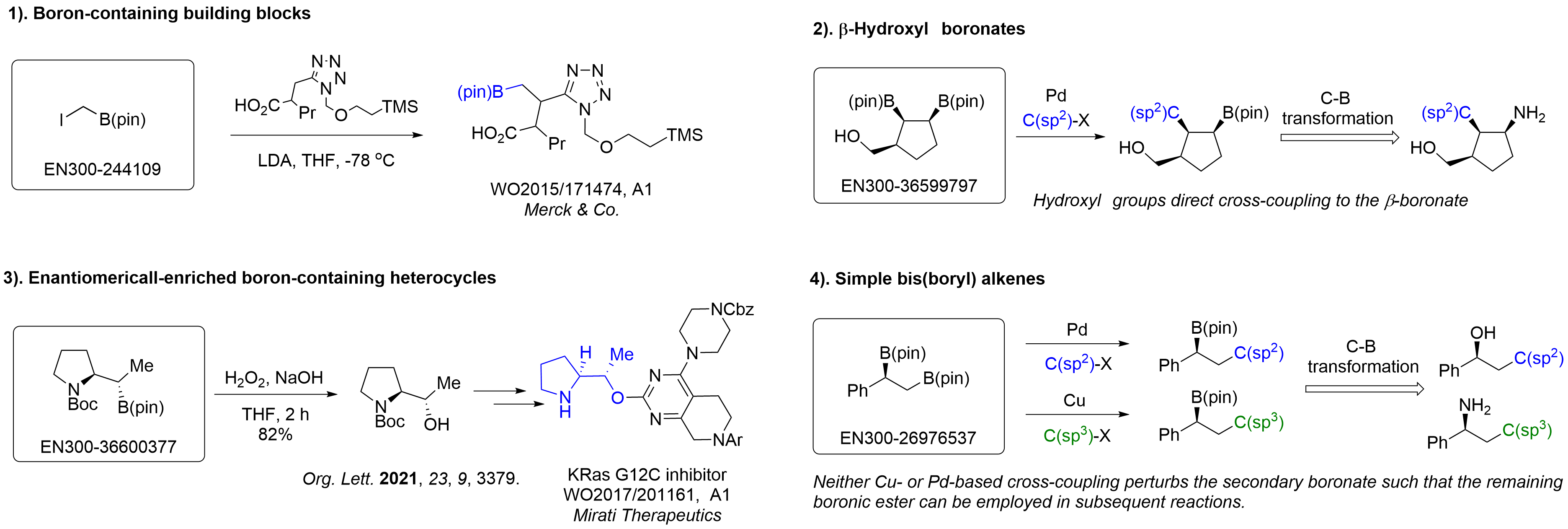
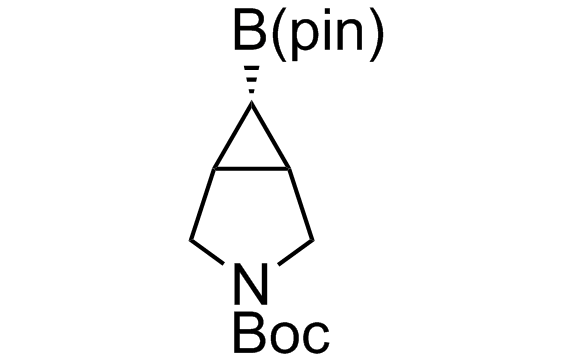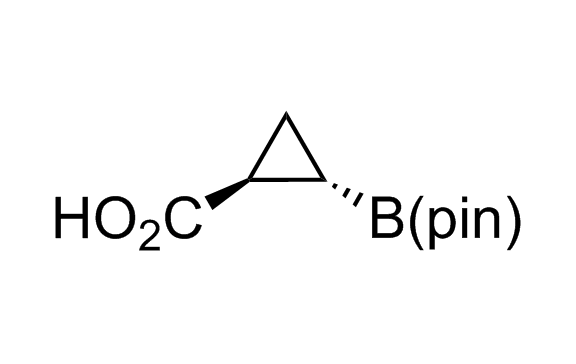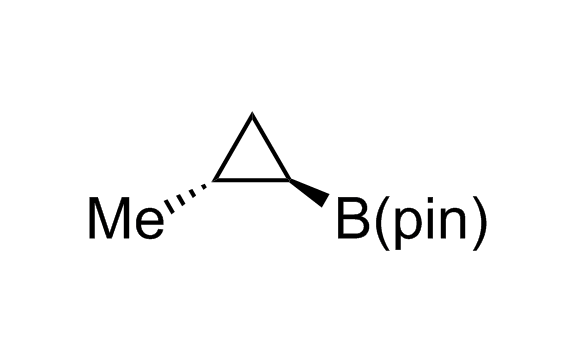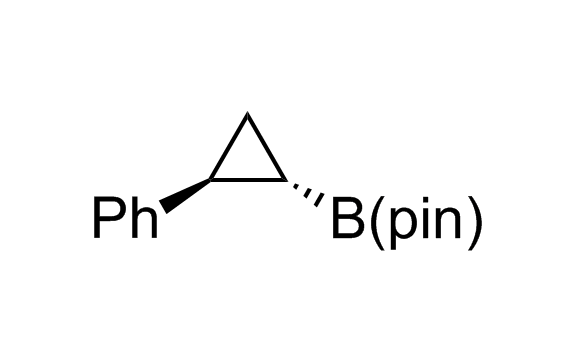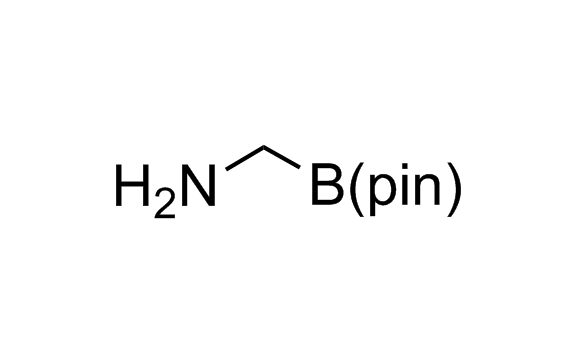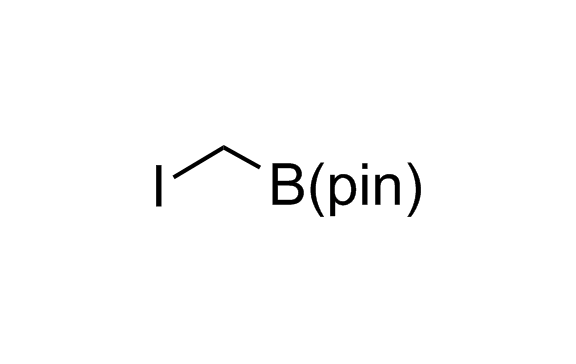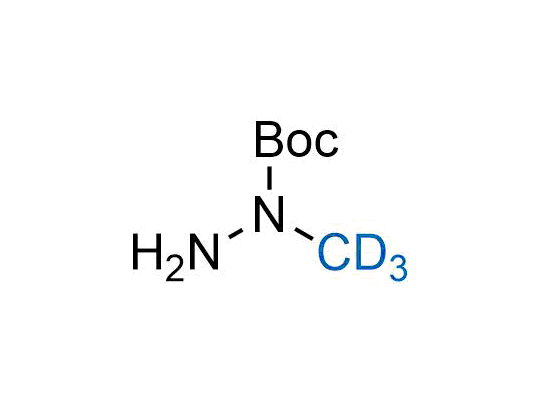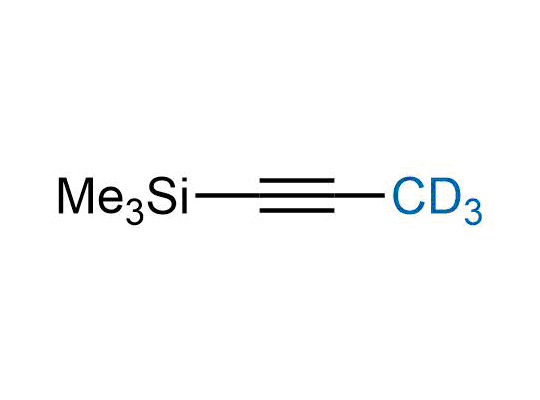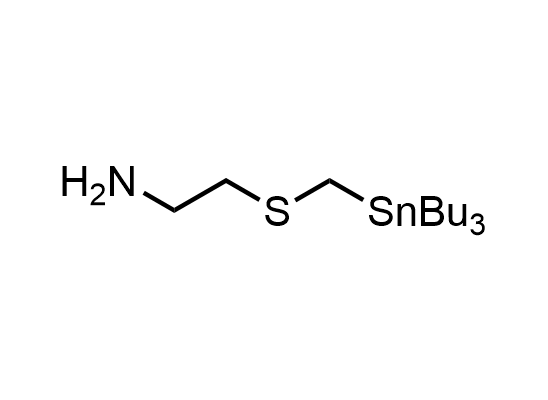Alkyl pinacol boronic esters have been routinely used for the synthesis of complex target molecules due to their low toxicity and stability. They can participate in cross-coupling and other boron-based transformations. It is expected that there may be a demand from the chemical industry for readily diversifiable chiral building blocks for use in construction of new chemical libraries. In this context, Enamine offers a library aliphatic pinacol boronates. We also have designed a library of pinacol boronates that are readily prepared in enantiomerically enriched fashion, and that can participate in cross coupling and other boron-based transformations.
Case studies
We offer
>100 unique boron-containing building blocks
MADE (Make-on-Demand) Building Blocks
These molecules can be synthesized upon request within 4-6 weeks.
Building blocks are the essence of our business and our main advantage used to build up all other products and services. Get all the compounds you need directly from the producer in one place, and embody your creativity with our building block collection.

MADE Building Blocks
Discover unexplored unique chemotypes with our MADE collection. MAke-on-DEmand (MADE) building blocks are a 1.28B catalog of reagents that can be synthesized within several weeks using short pre-validated reaction sequences, our chemical experience, and starting materials from our stock.
Fast MADE Building Blocks
To save your time and efforts, we conveniently categorized the entire Enamine's building blocks collection by chemical functional groups: carboxylic acids, primary and secondary amines, boronic compounds, etc. Download latest selections and special subsets as separate SD files.
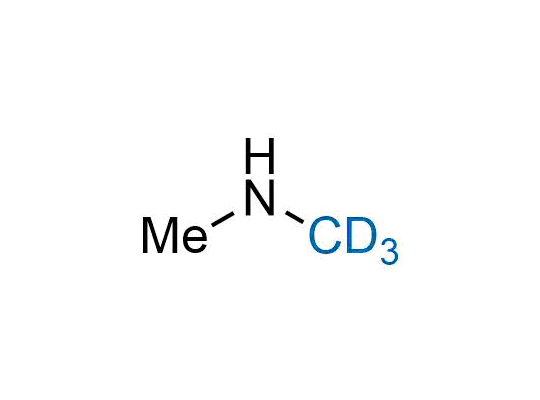
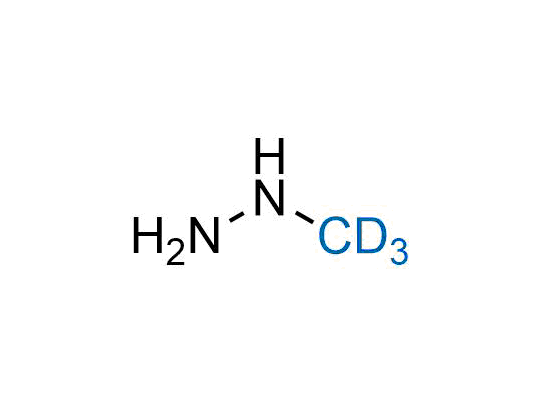
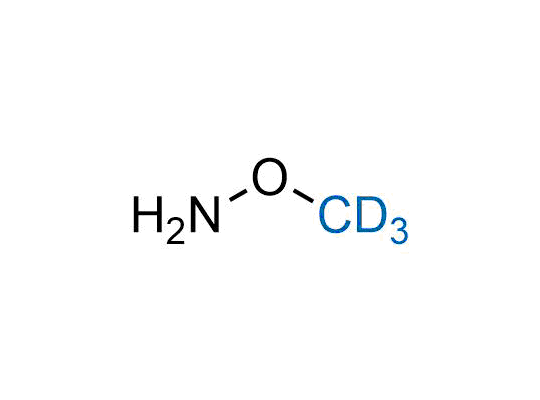
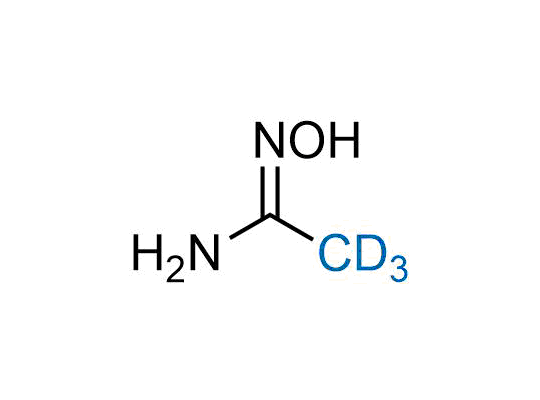
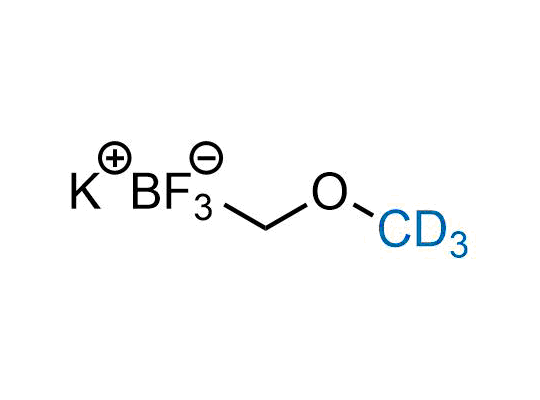
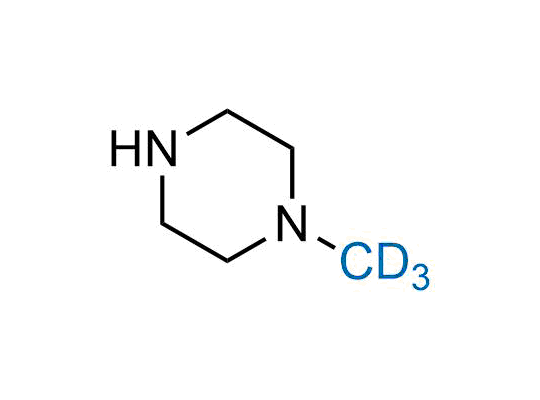
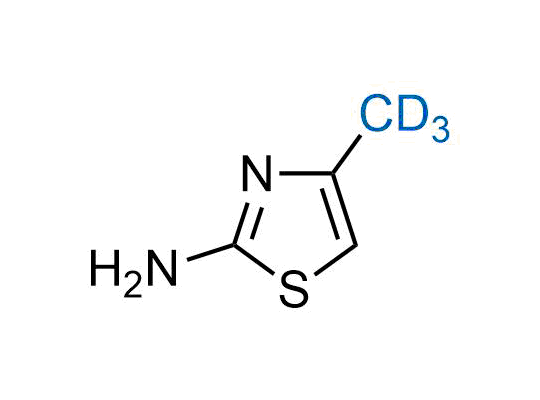
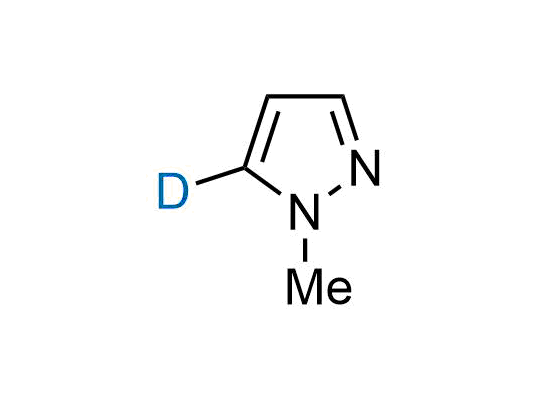
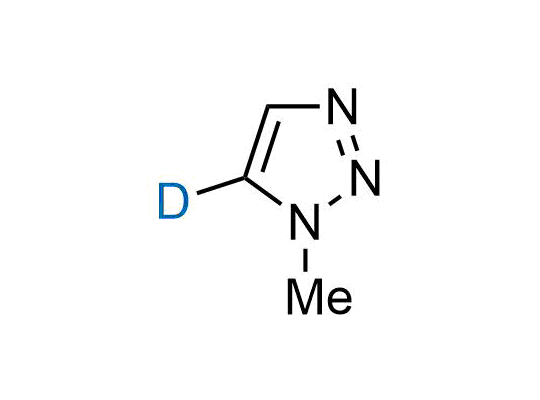
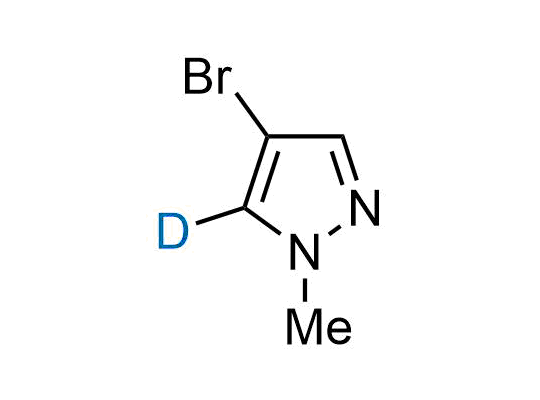
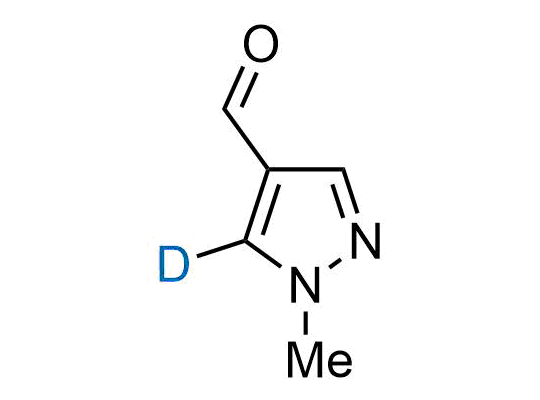
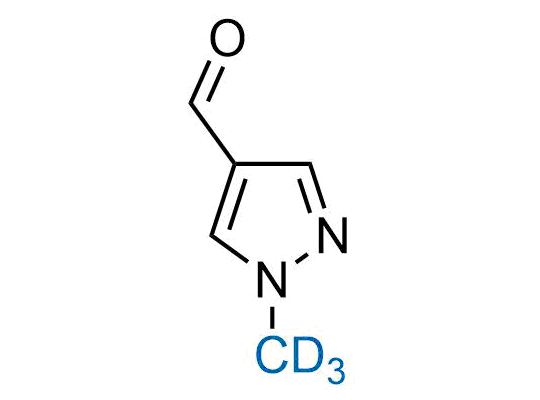
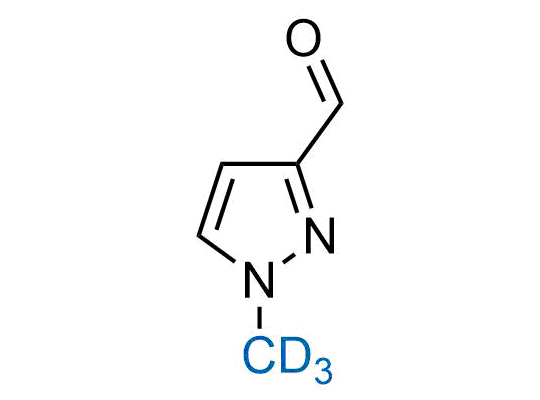
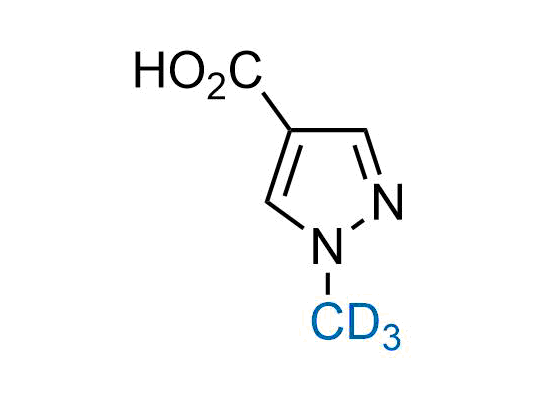
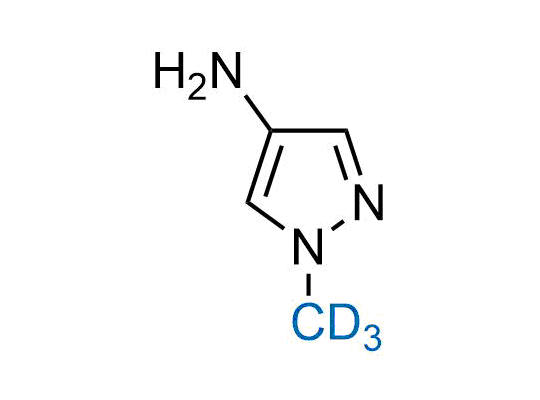
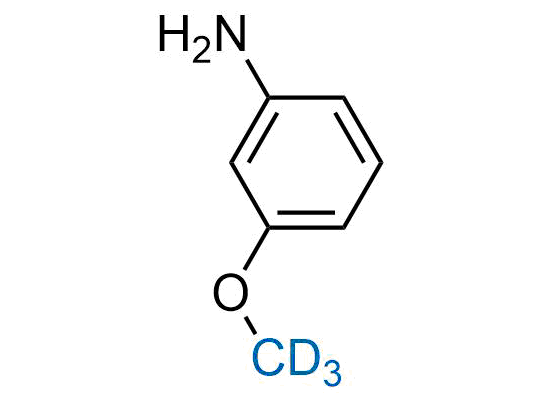
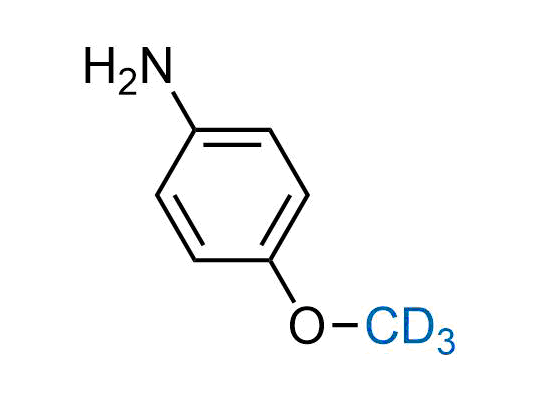
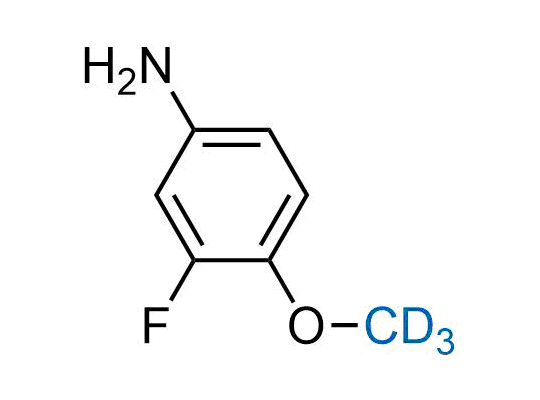
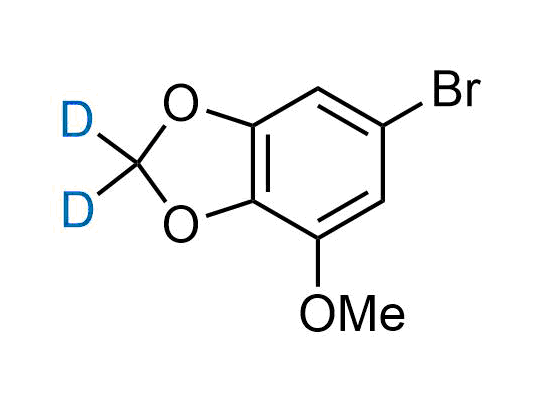
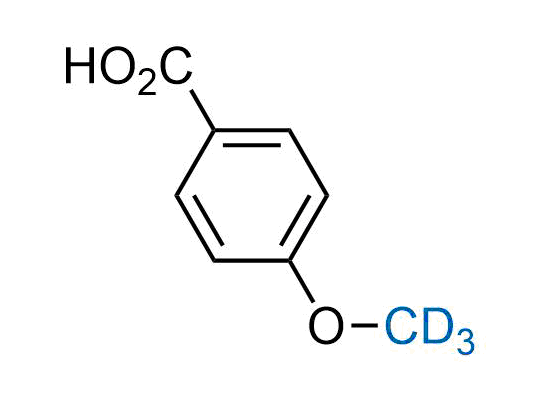
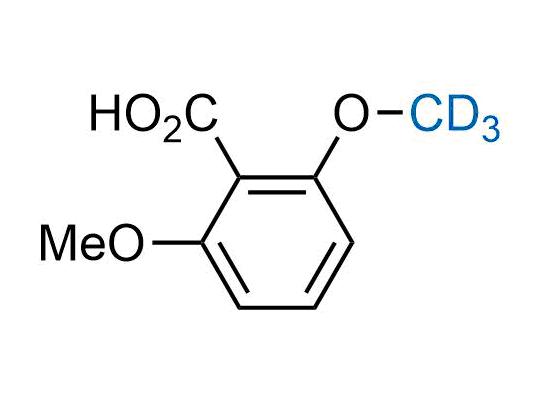
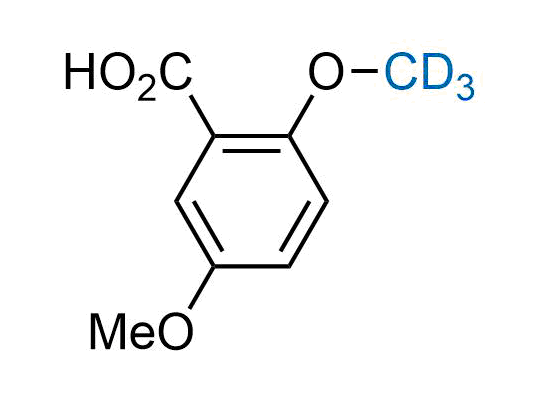
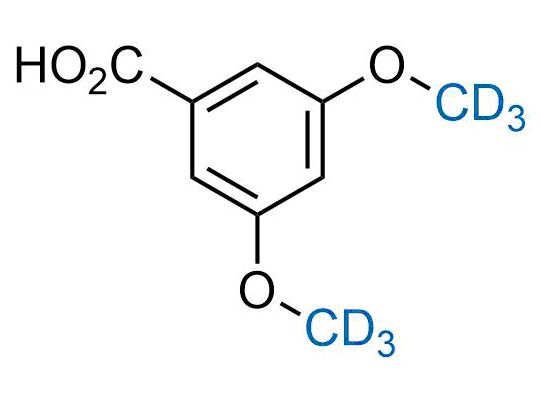
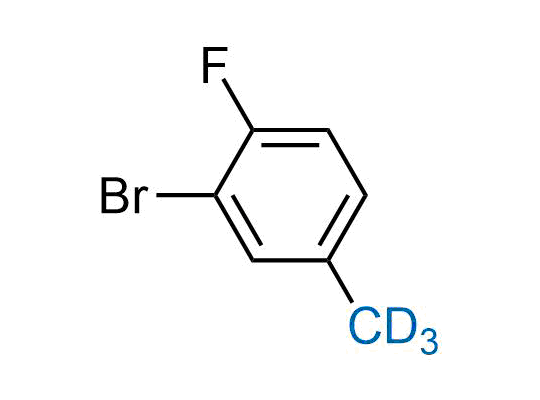
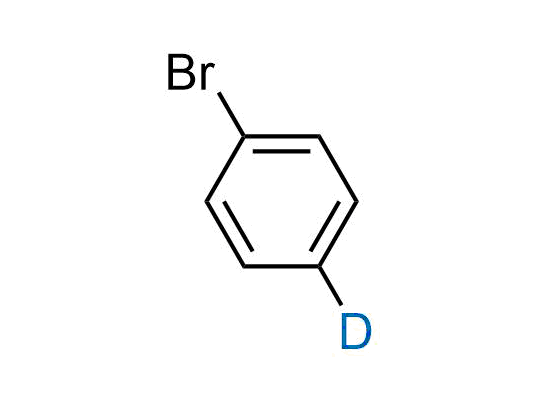
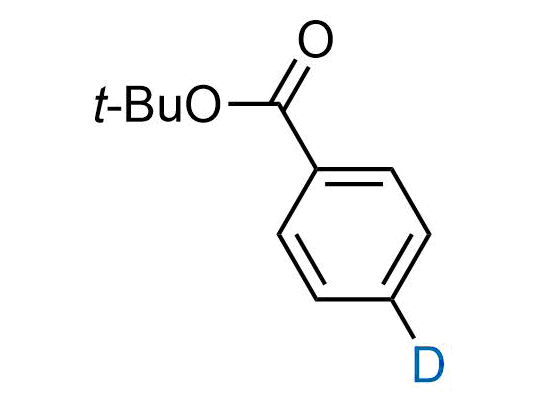
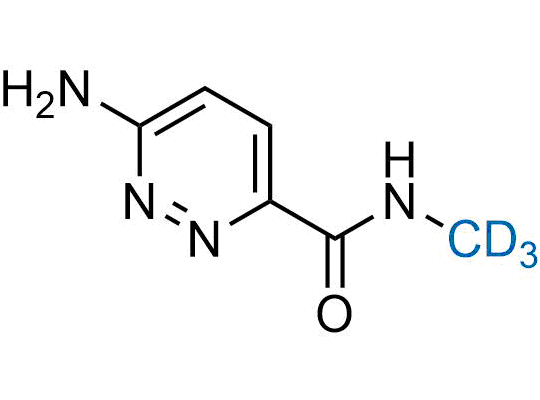
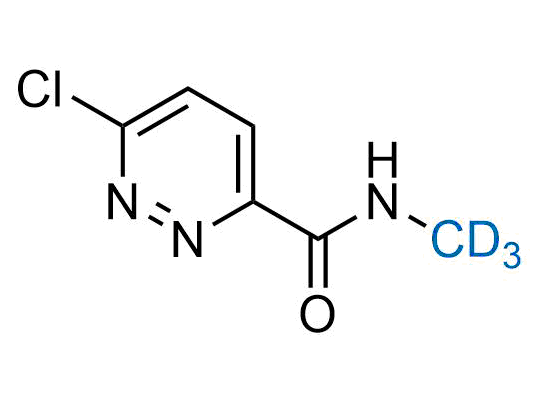
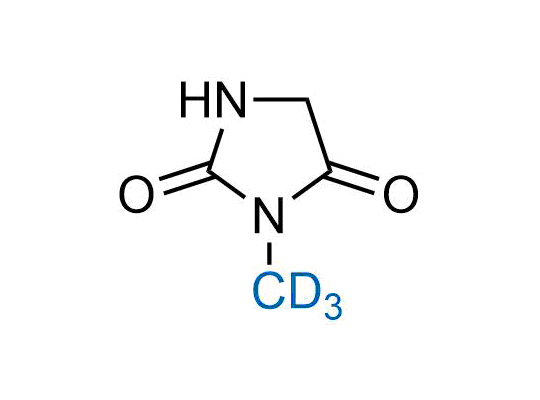
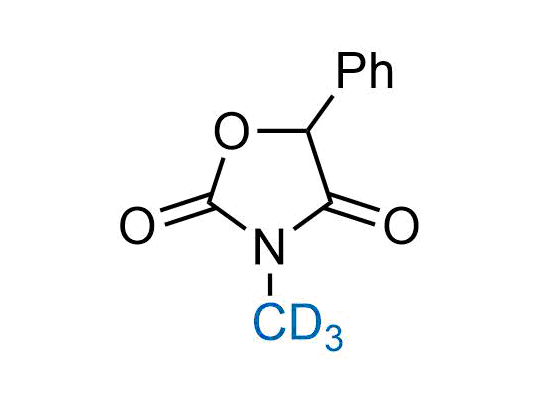
In 2022, the FDA approved the second deuterium-containing drug. Deuterium exchange has proven effects on drug pharmacokinetics, inhibiting demethylation, reducing oxidation, and slowing racemization. This leads to prolonged drug exposure, lower doses, and fewer side effects. Deuterium has improved many existing drugs, making it important to consider deuterated variants when filing patents for new pharmaceuticals.
Enamine is the recognized leader in the synthesis of advanced building blocks for discovery chemistry. Our chemists have synthesized a sizable collection of deuterium-containing building blocks to design new therapeutics.
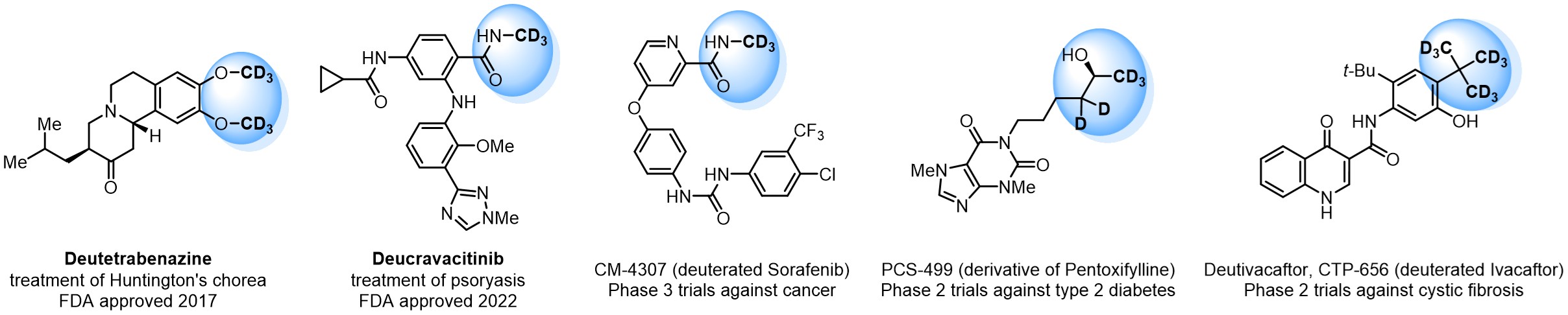
Case studies
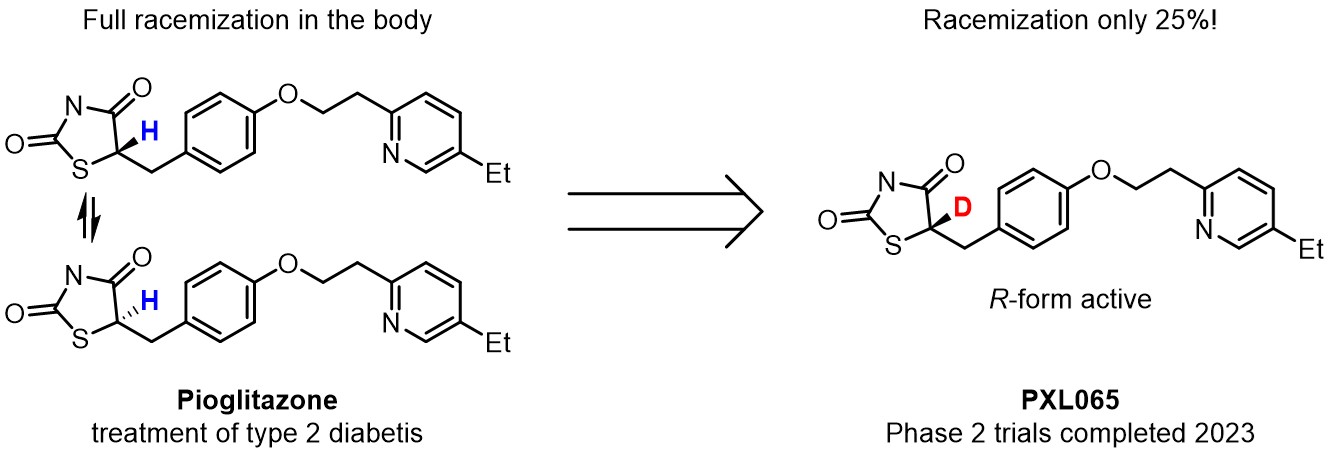
Pioglitazone has been in medical use against type 2 diabetes since 1999. The substance was administered as racemate, as it undergoes full racemization in the patient’s body. However, the incorporation of deuterium to the molecule significantly decreased racemization, resulting in a ratio ~4:1. Deuterium allowed to administer the substance in the R‑chiral form, leading to a notable reduction in side effects.
Download SD file
Download PDF file
We offer
Over 50 deuterium-containing building blocks on a gram scale.
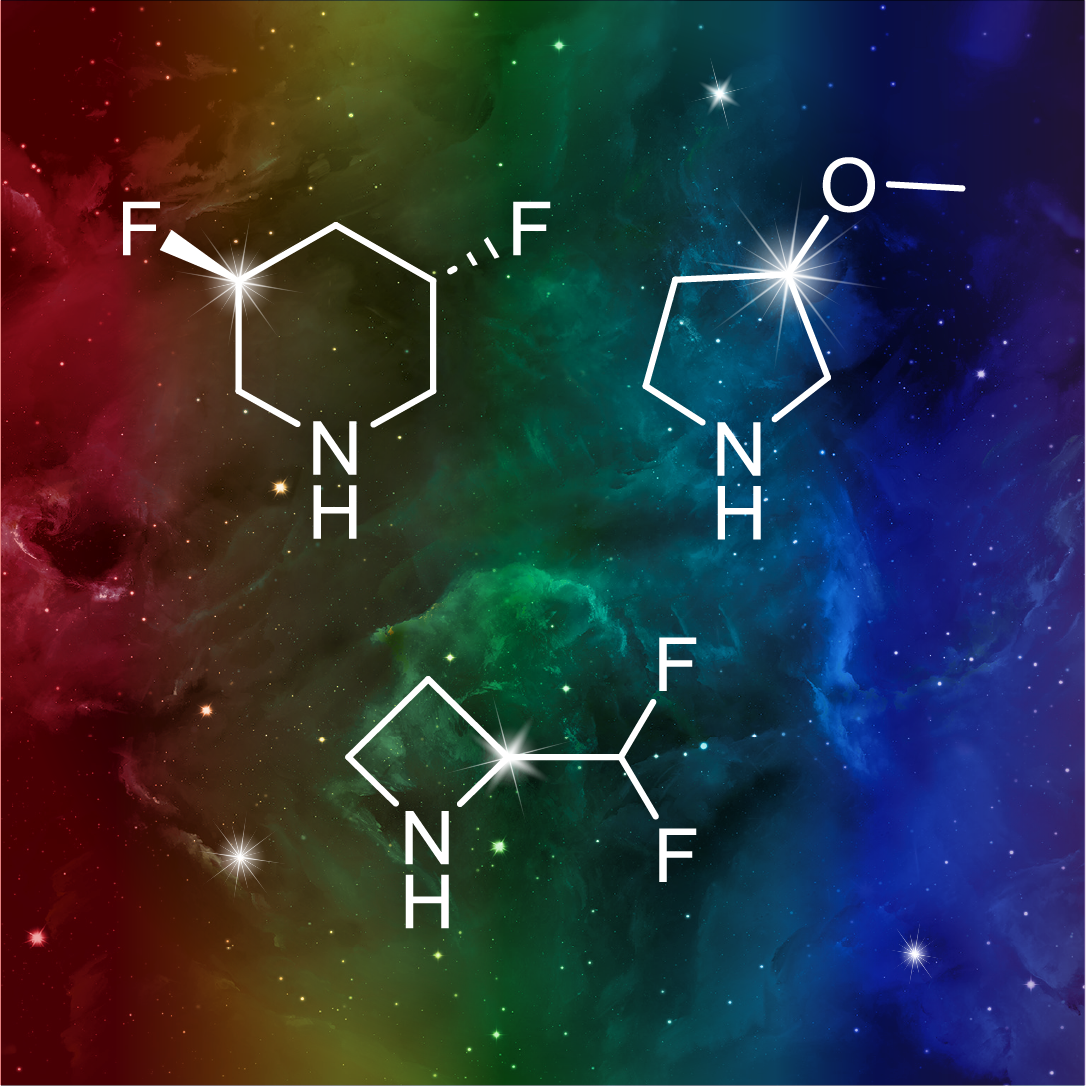
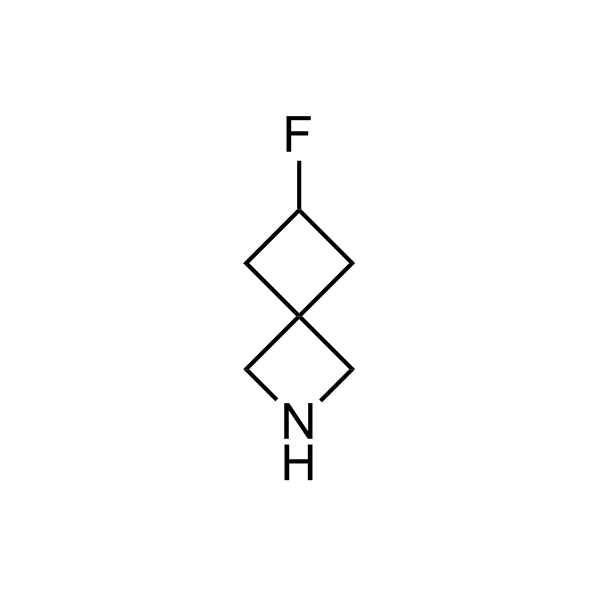
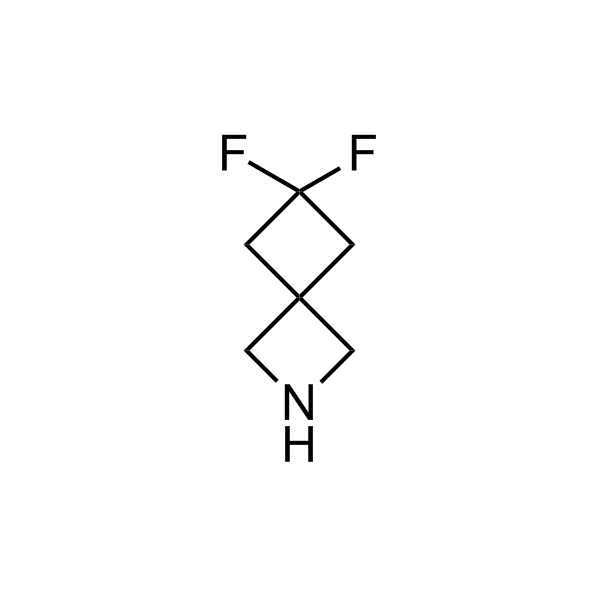
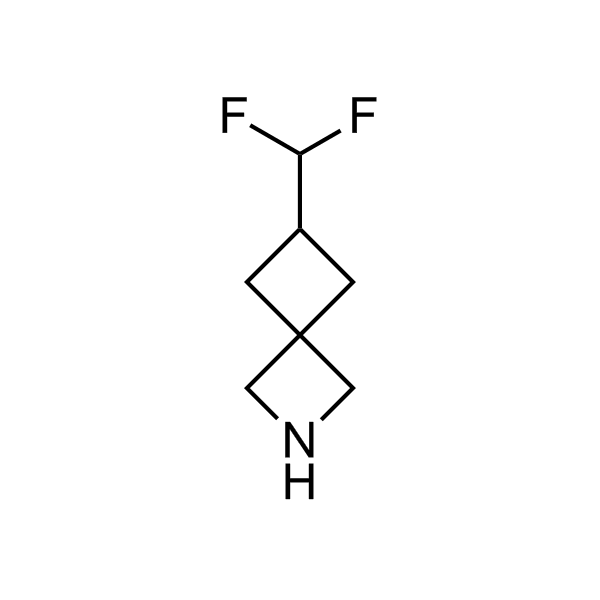
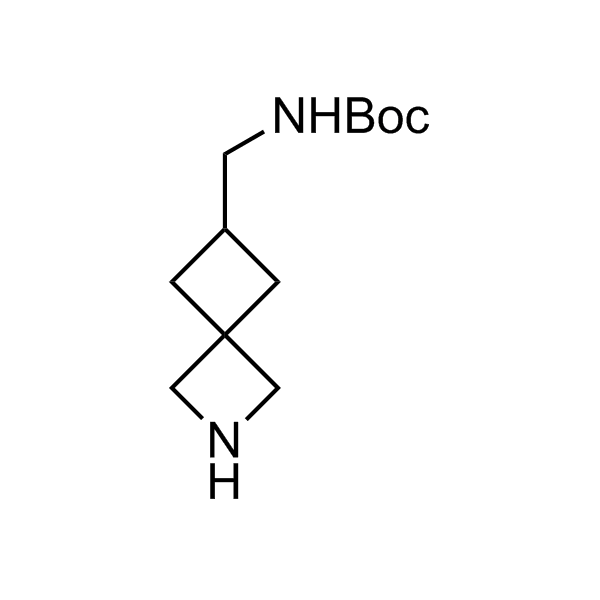
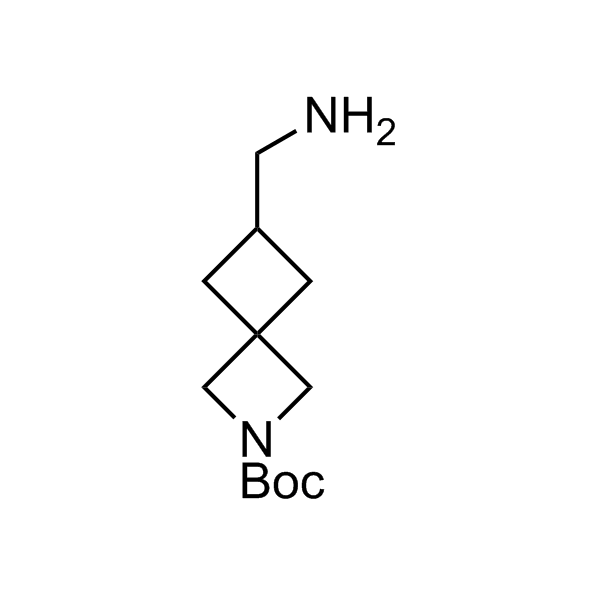
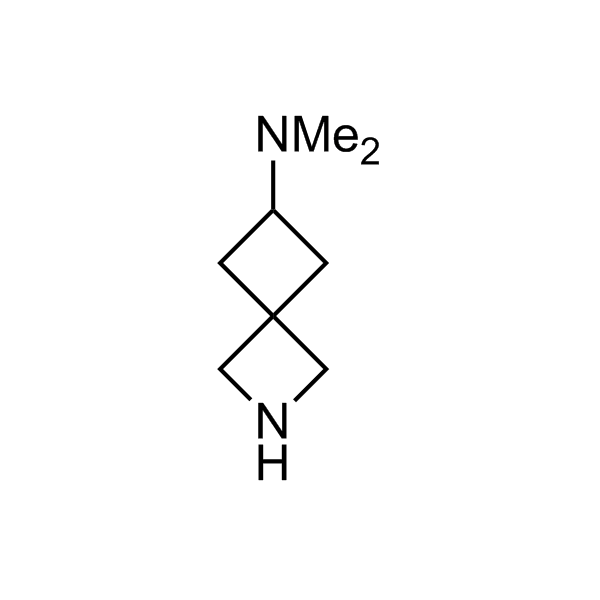
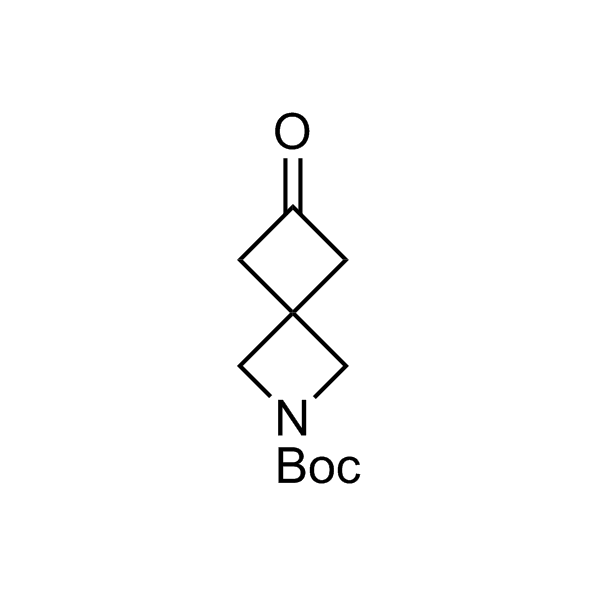
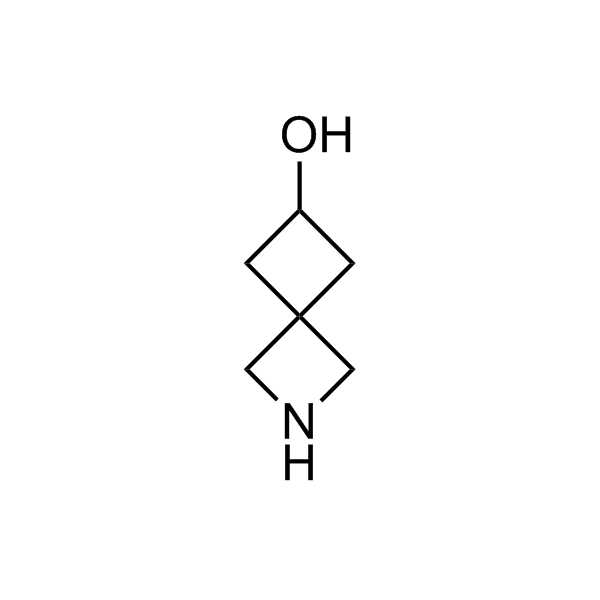
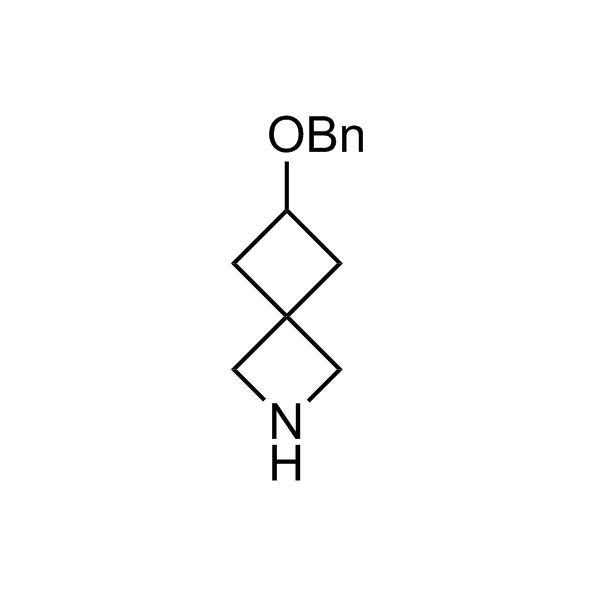
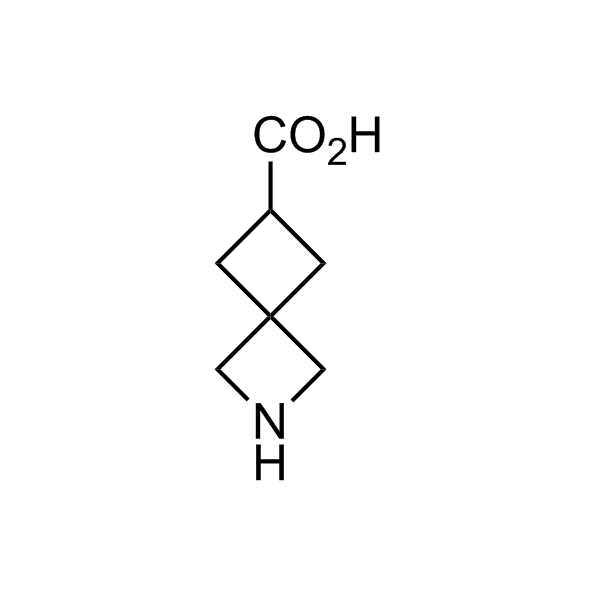
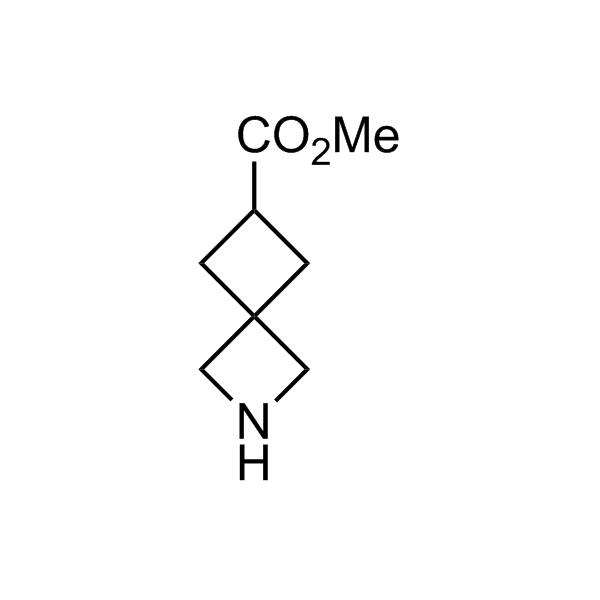
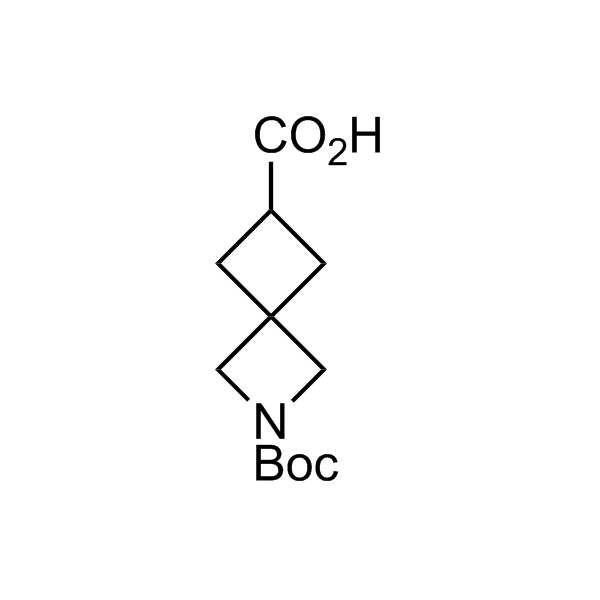
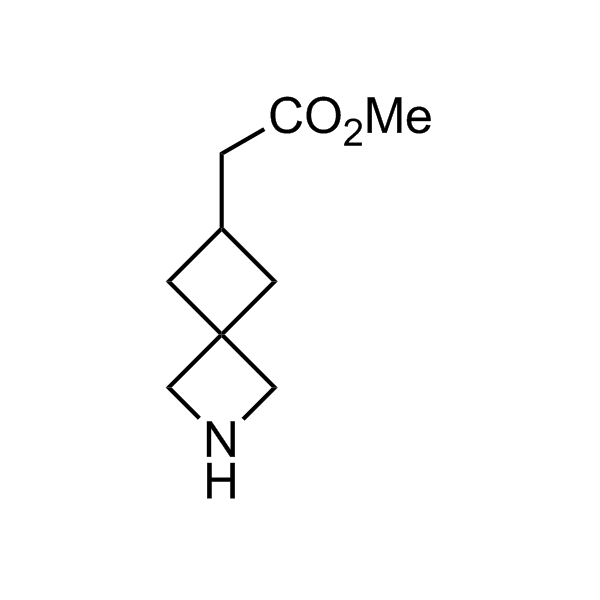
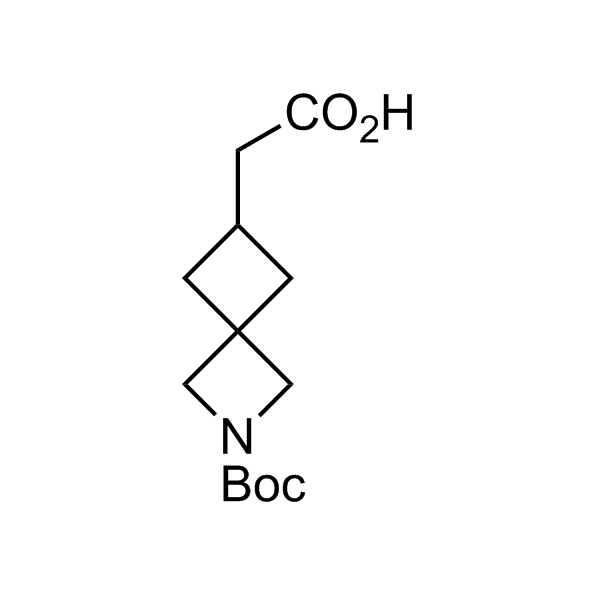
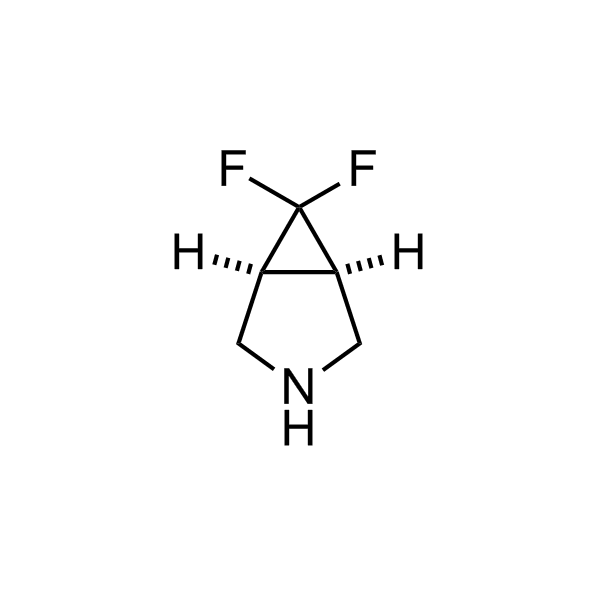
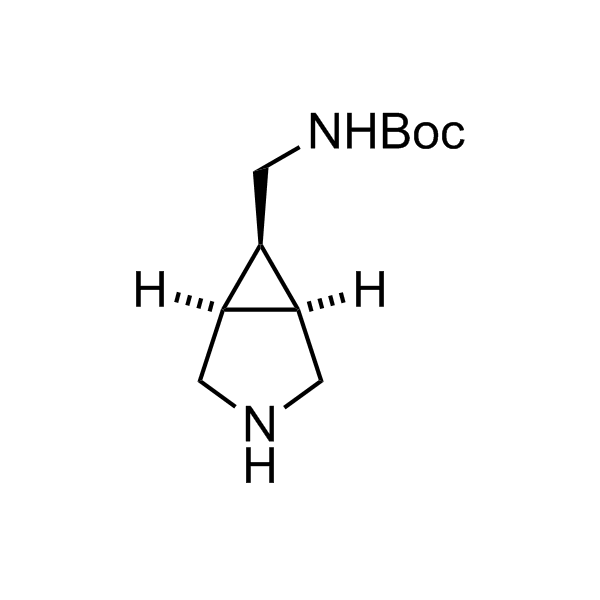
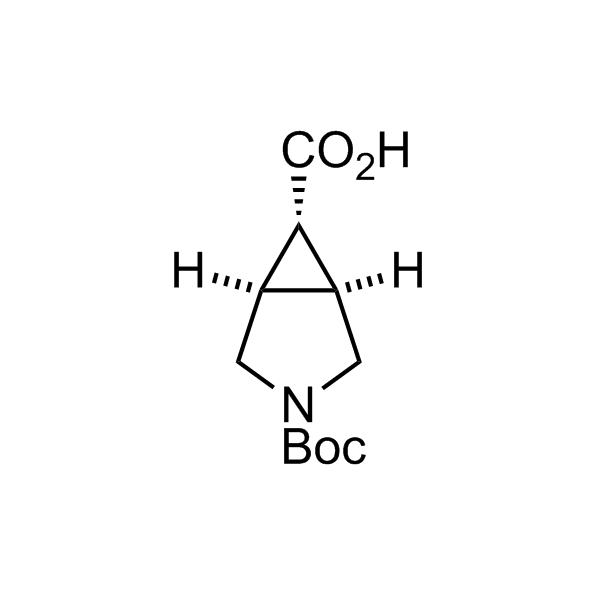
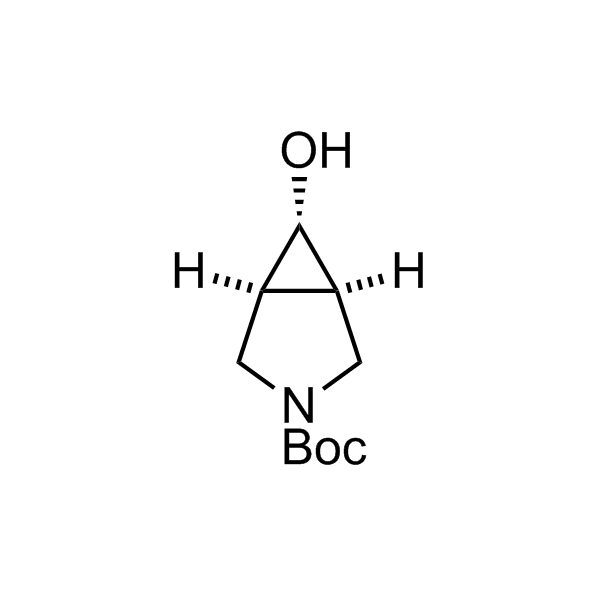
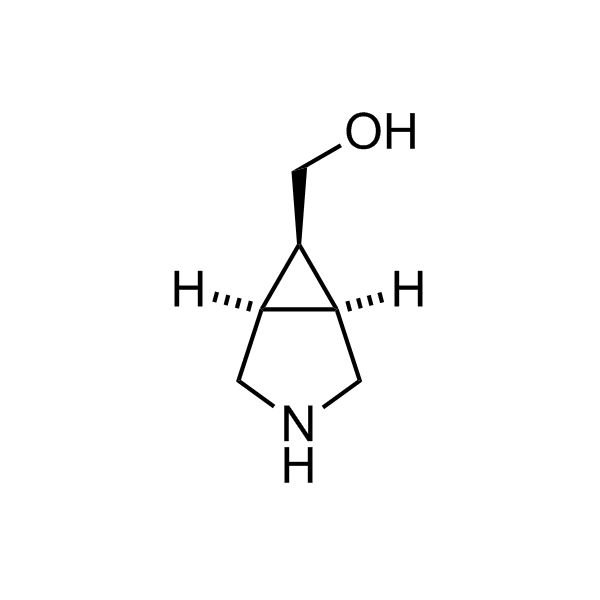
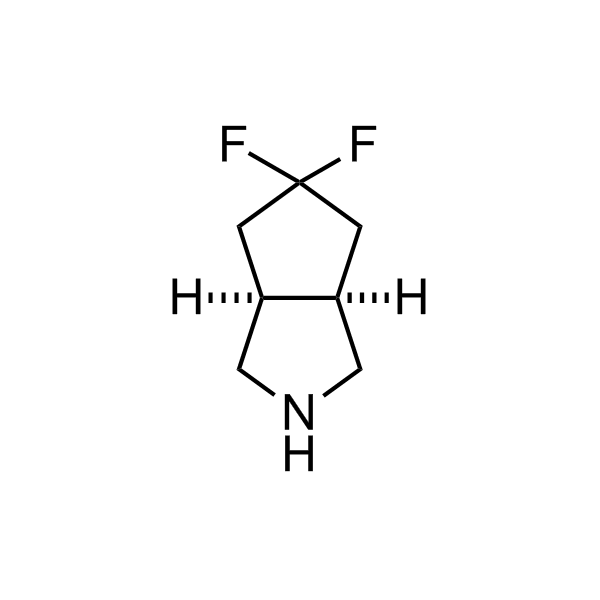
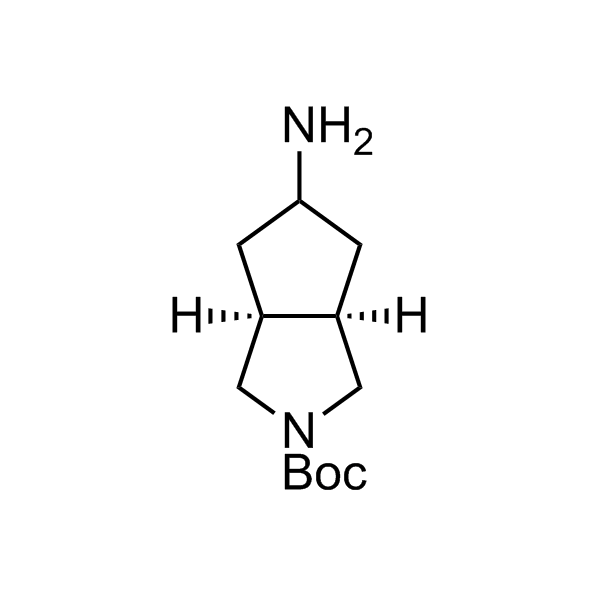
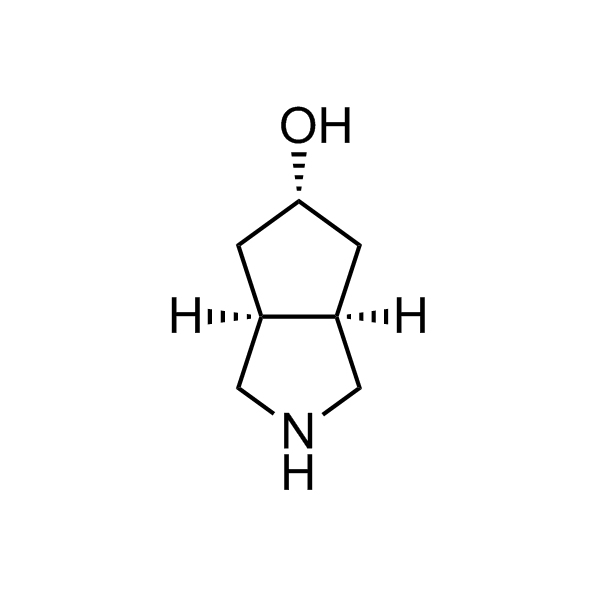
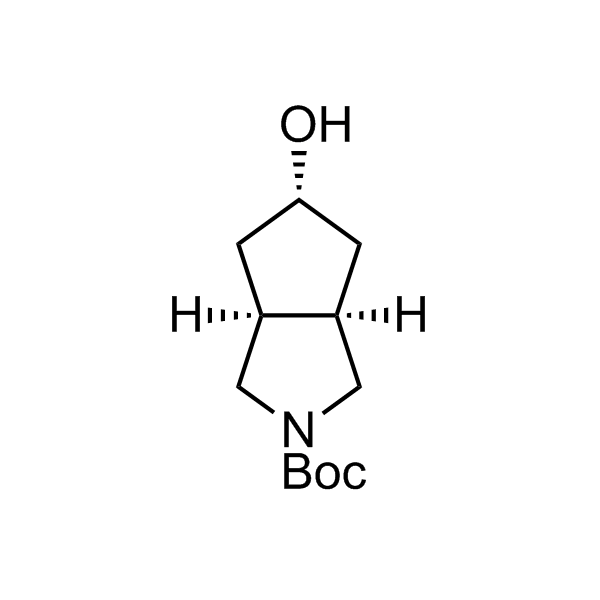
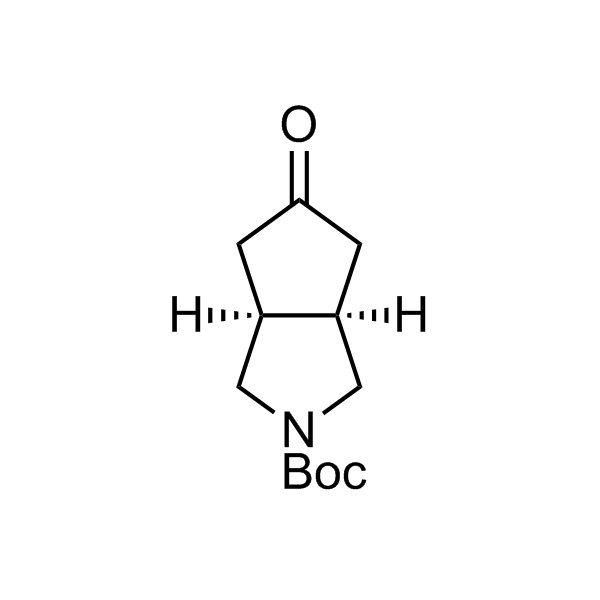
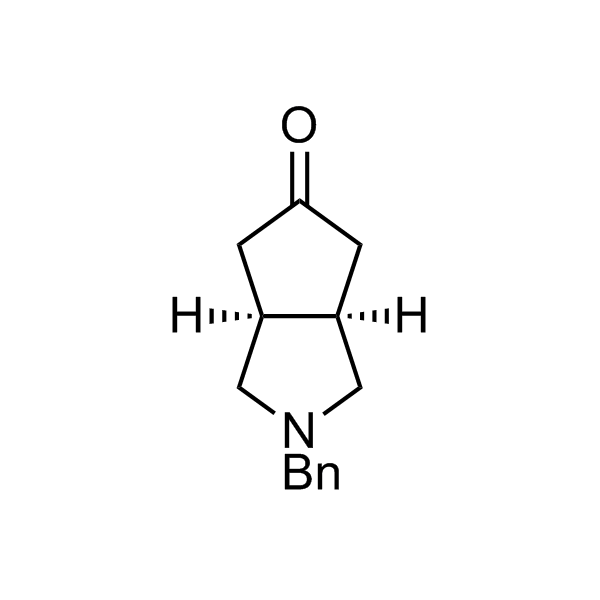
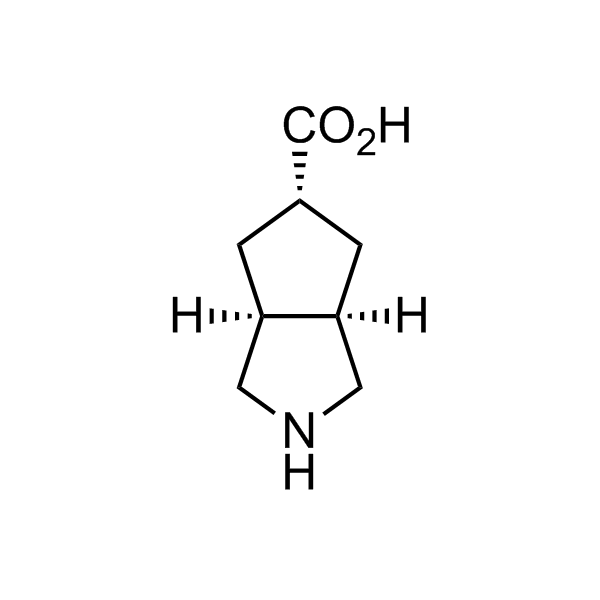
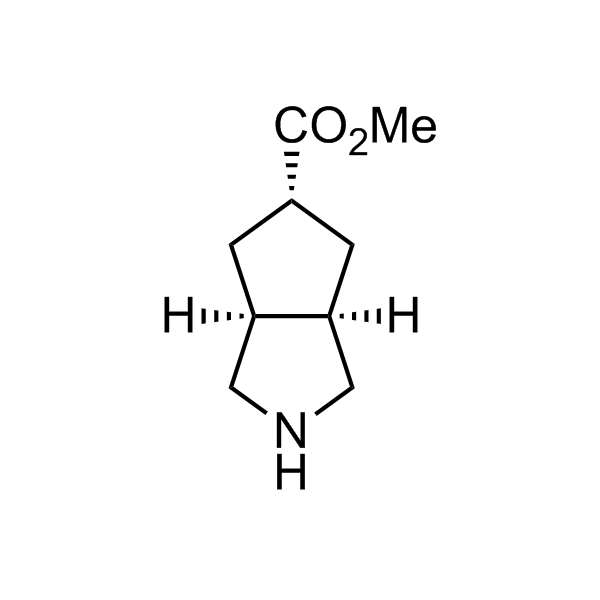
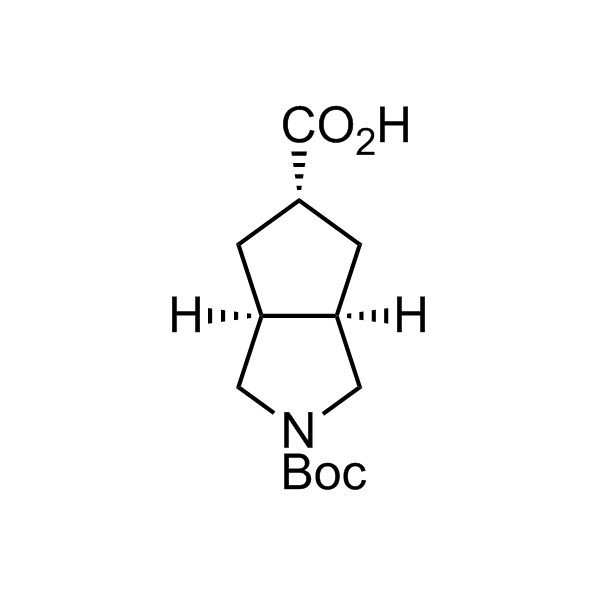
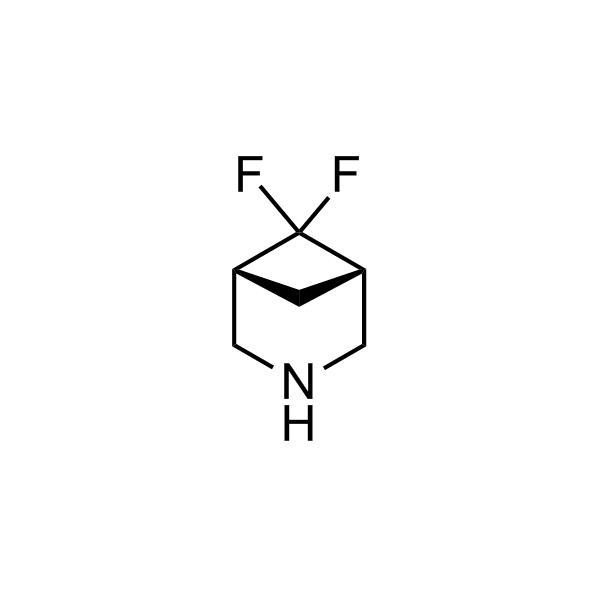
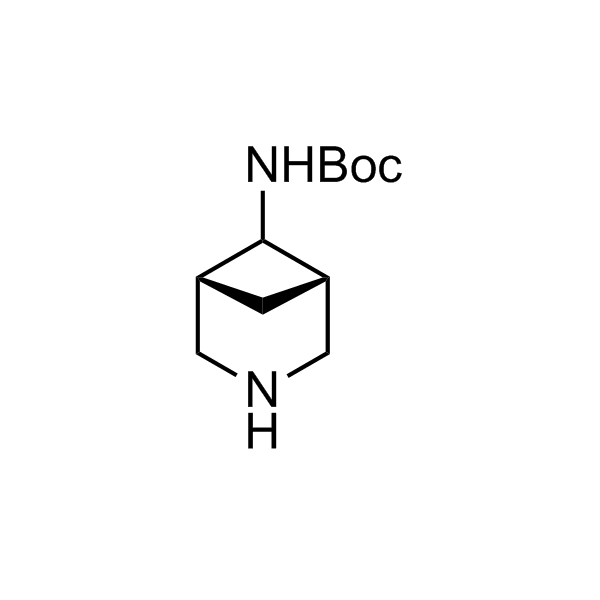
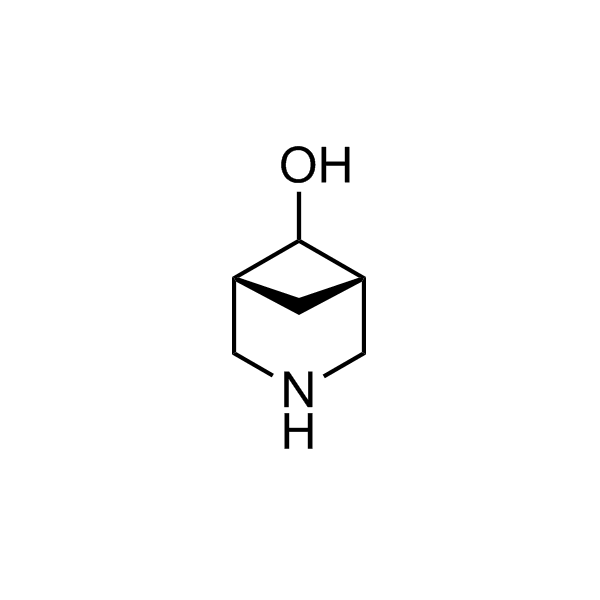
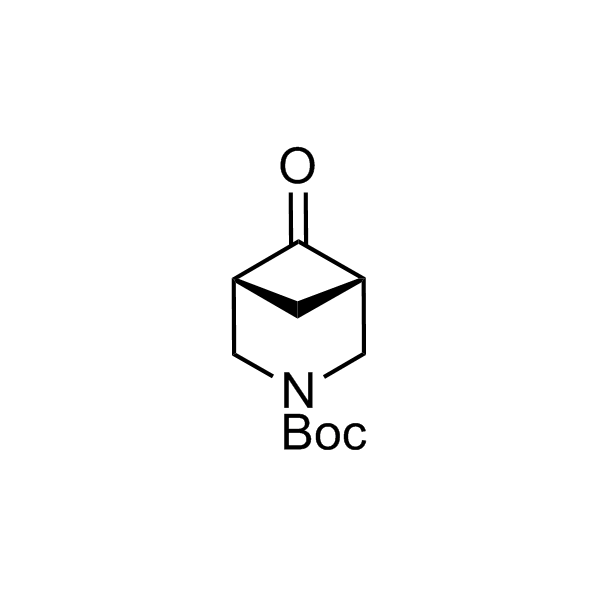
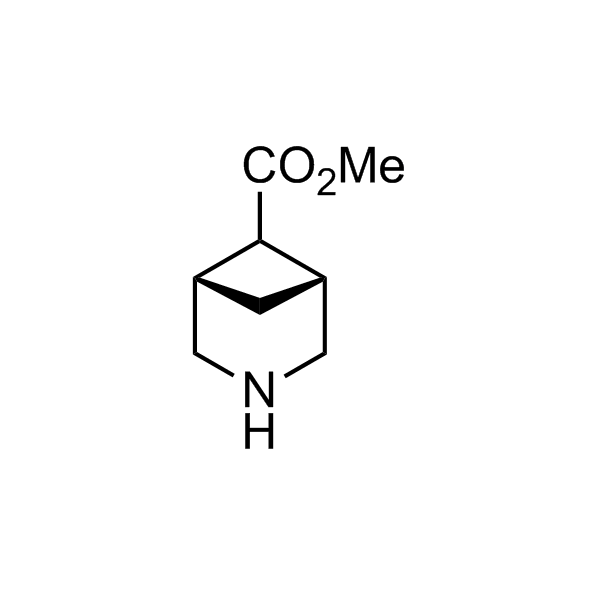
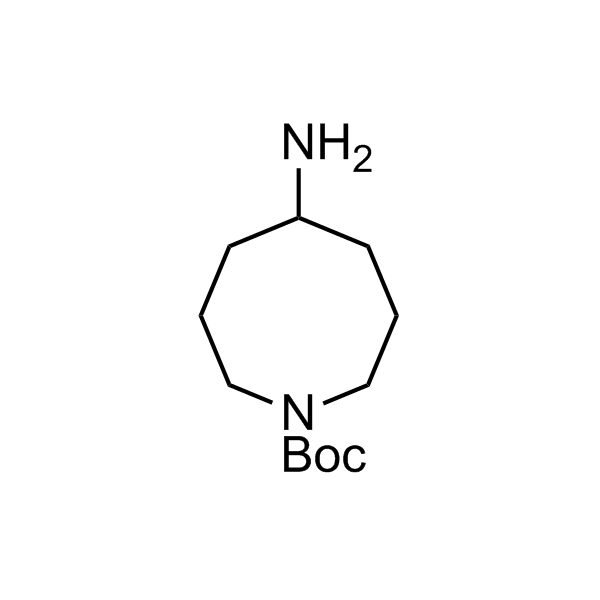
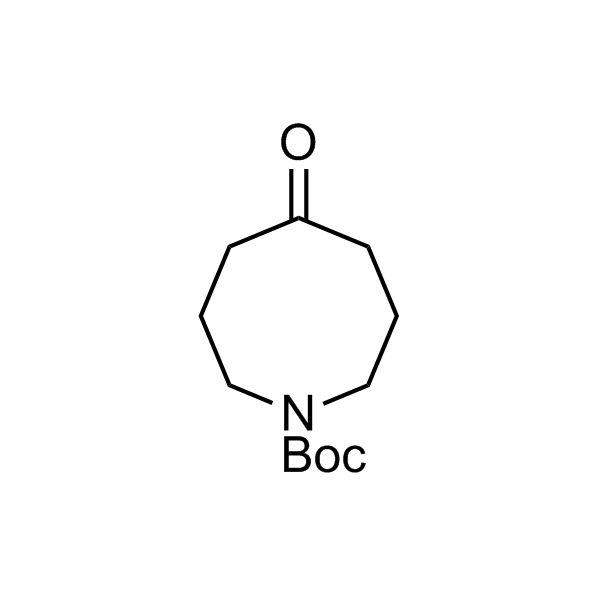
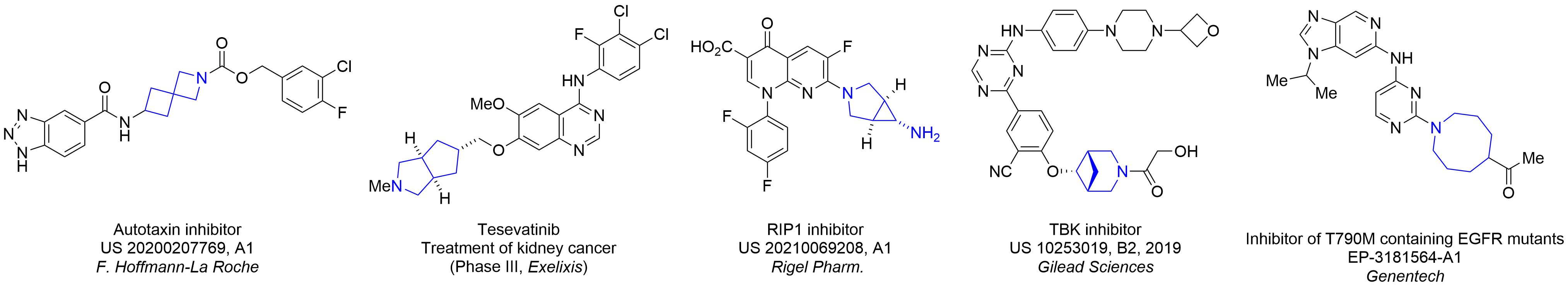
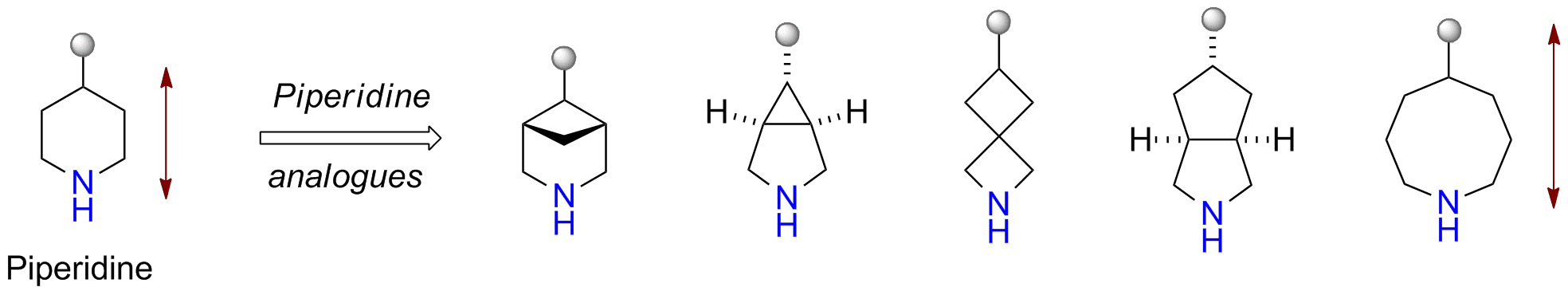
More than 70 FDA-approved drugs contain the piperidine moiety. Piperidine-based analogues may advantageously alter important pharmacokinetic properties such as lipophilicity and metabolic stability when grafted onto molecular scaffolds. Synthetic strategies for setting new spirocyclic, fused and bridged sp3-rich scaffolds are in high demand in the medicinal chemistry community. Herein, we have designed and synthesized a library of piperidine analogues for drug design.
Design
Download SD file
Download PDF file
We offer
>100 unique piperidine analogues on a 5-50 g scale from stock.
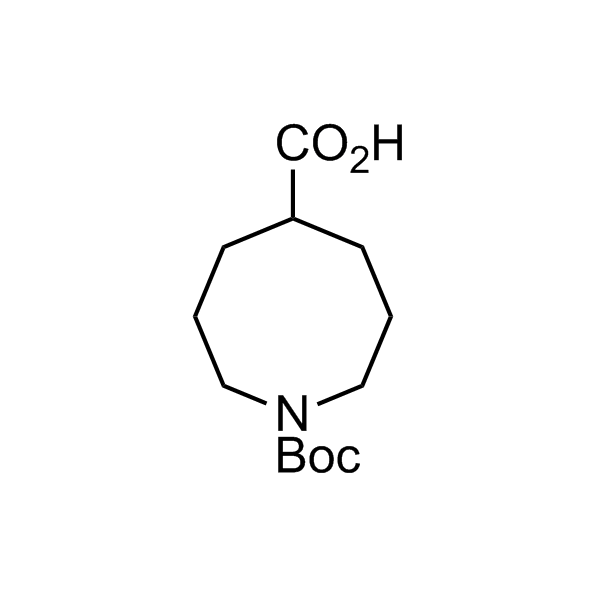
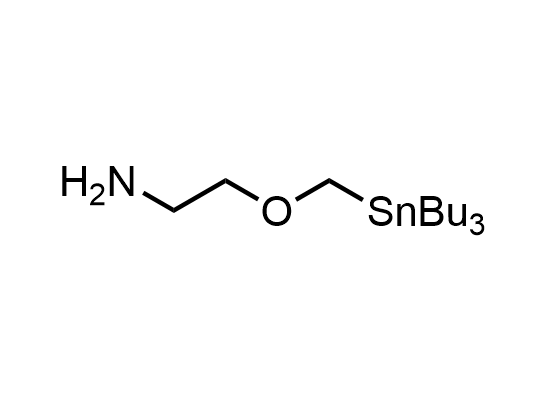
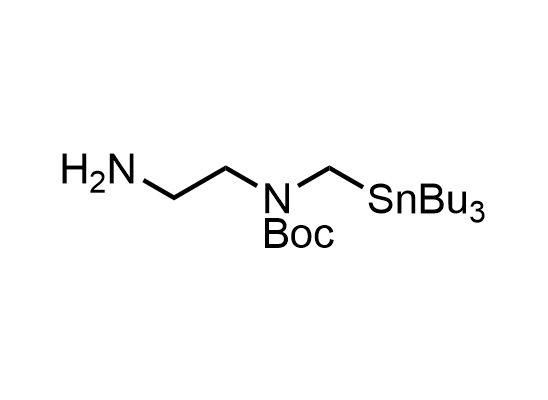
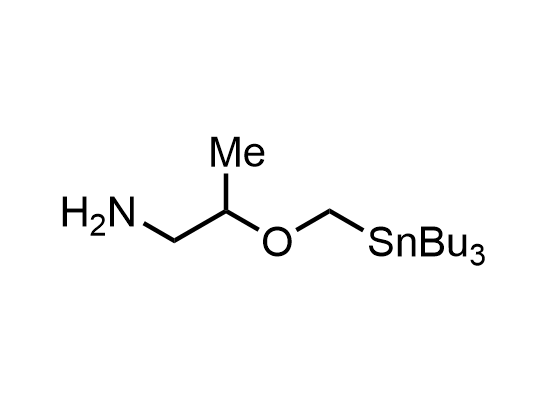
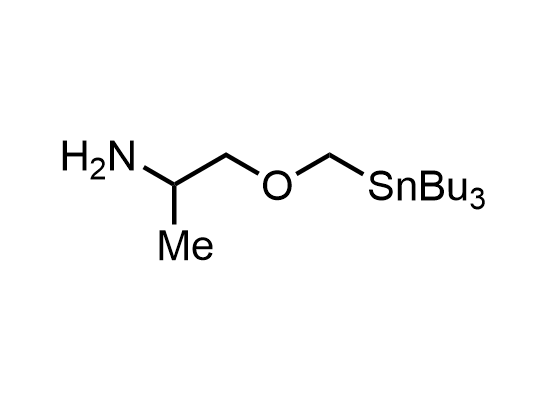
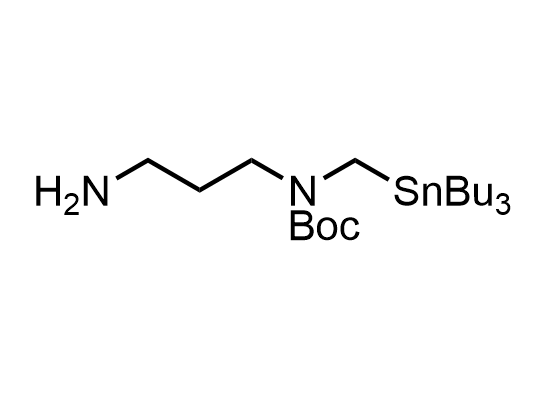
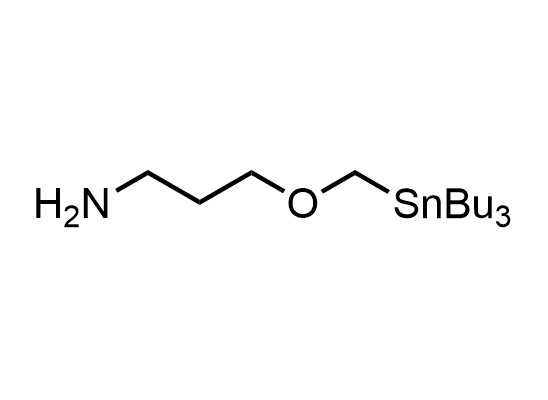
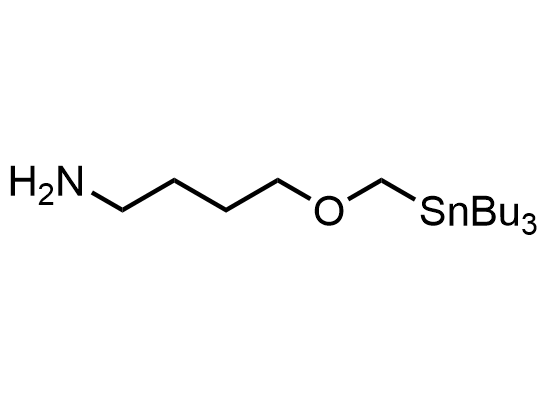
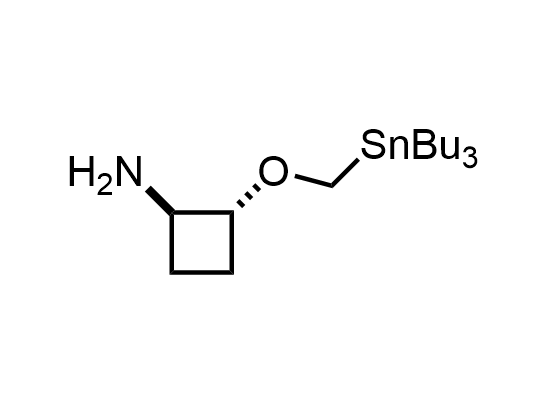
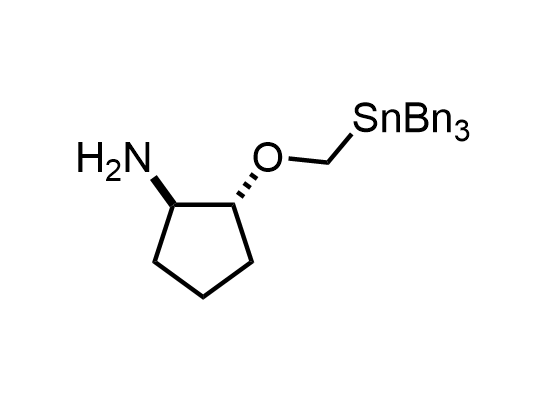
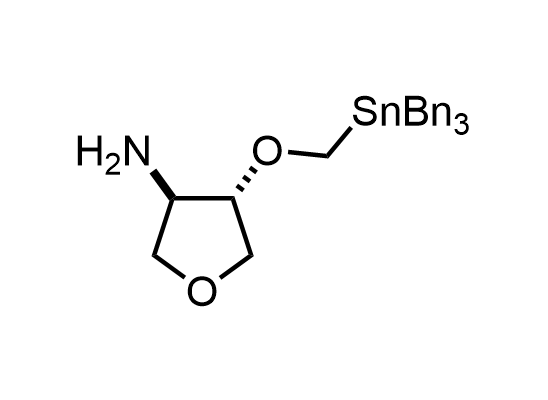
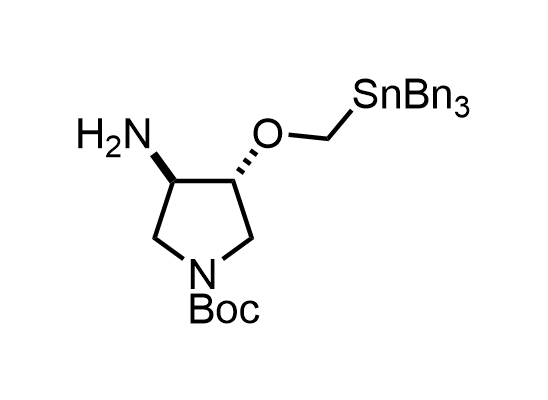
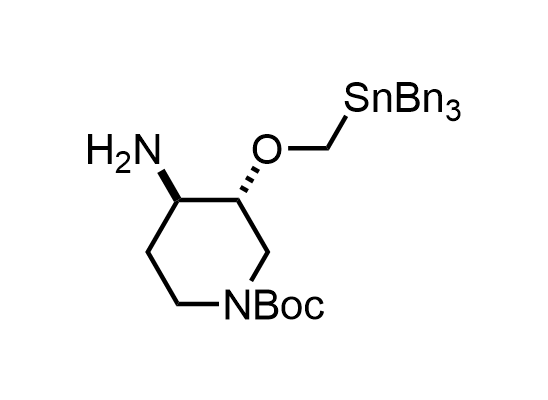
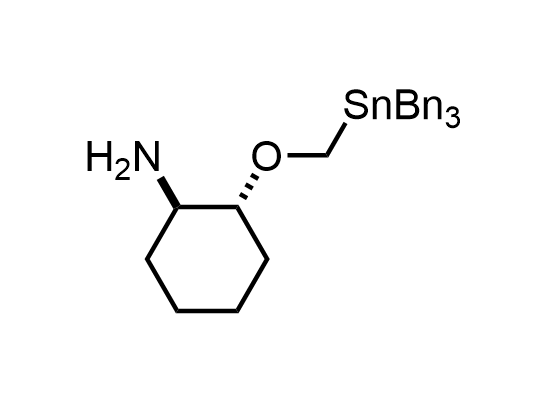
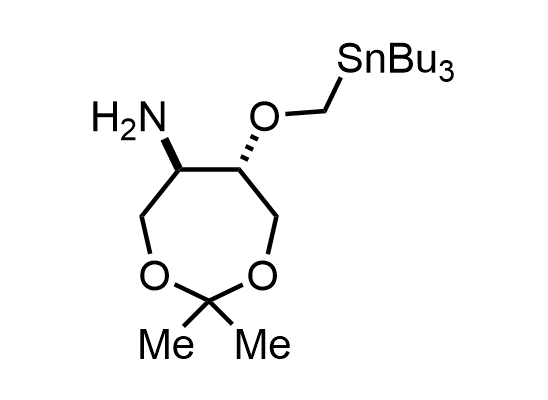
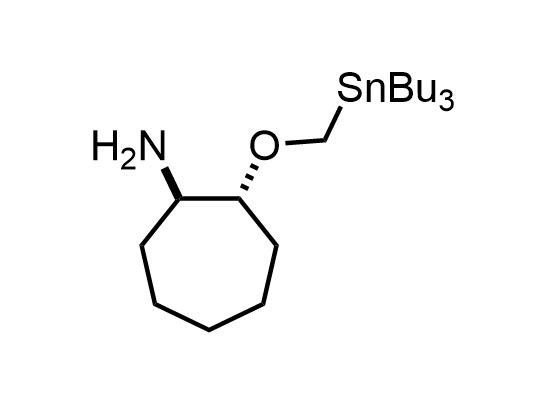
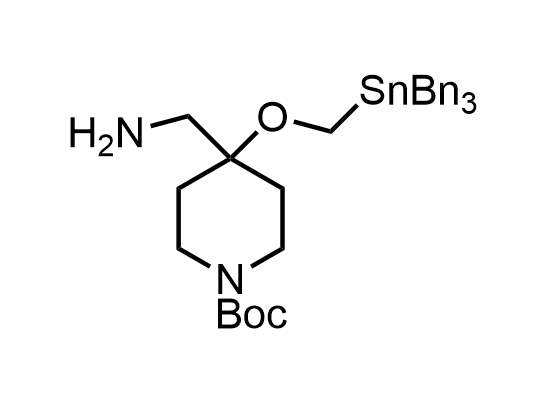
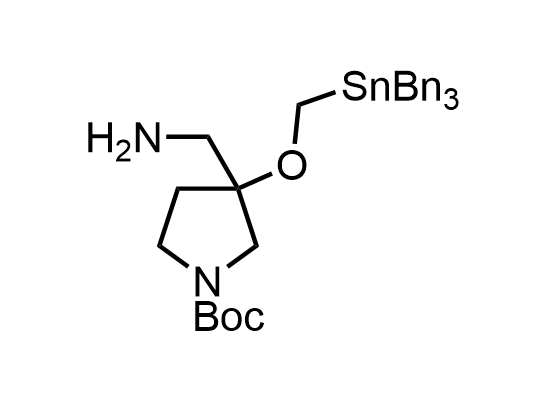
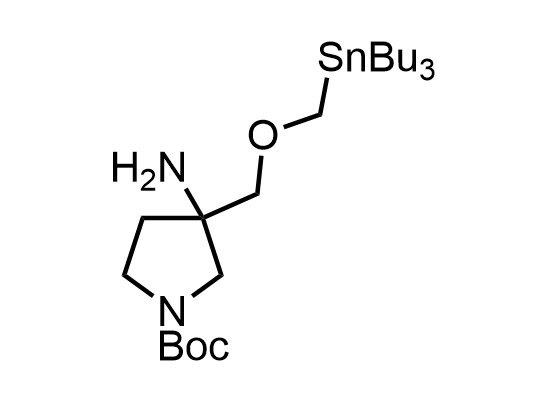
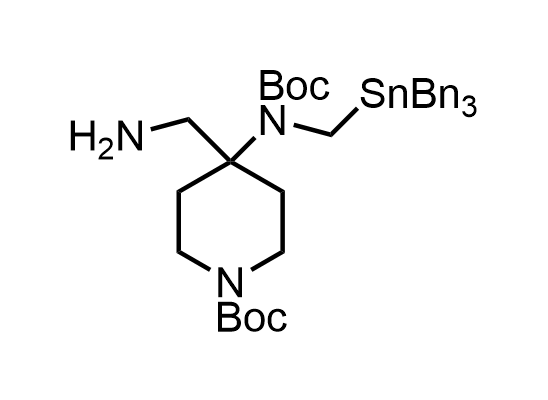
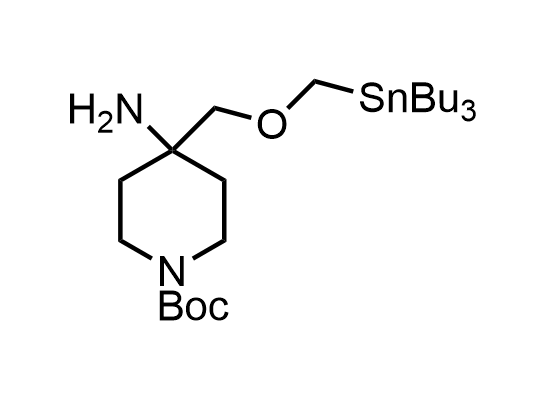
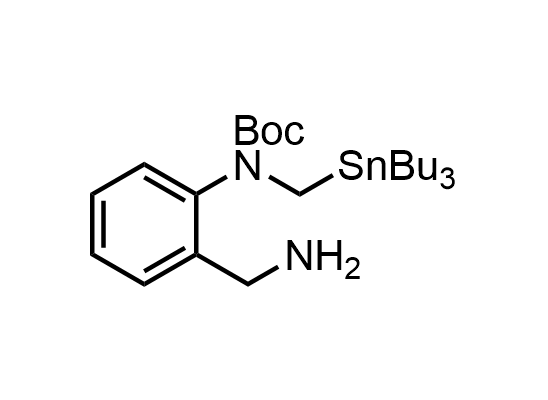
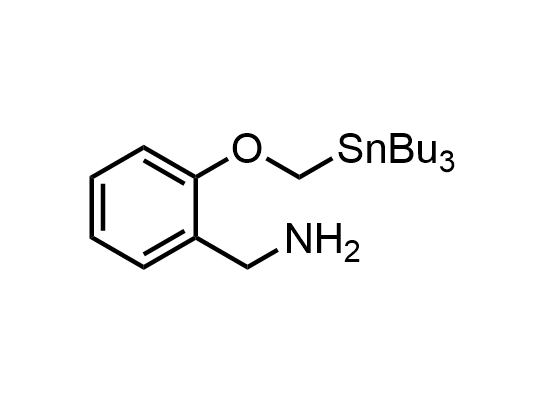
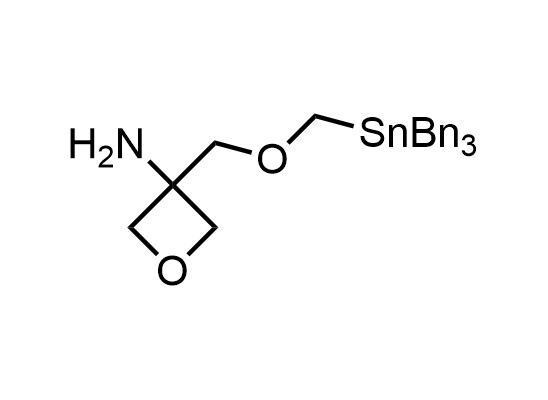
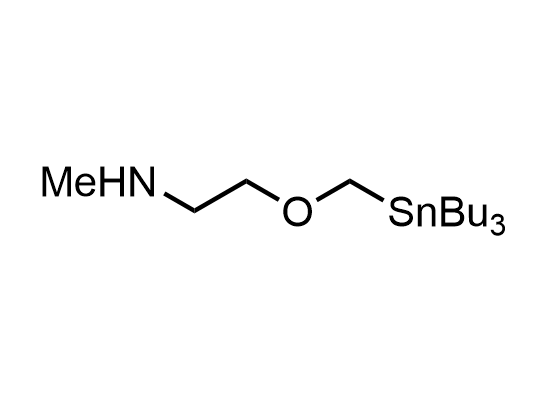
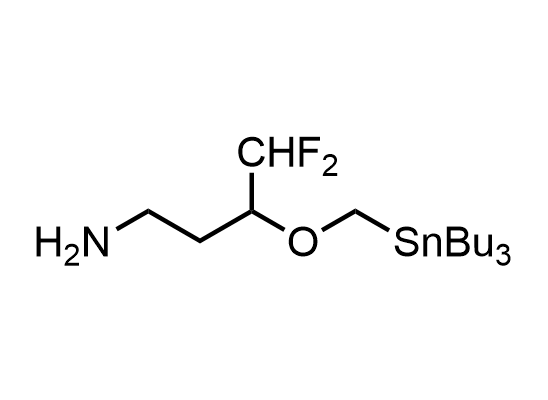
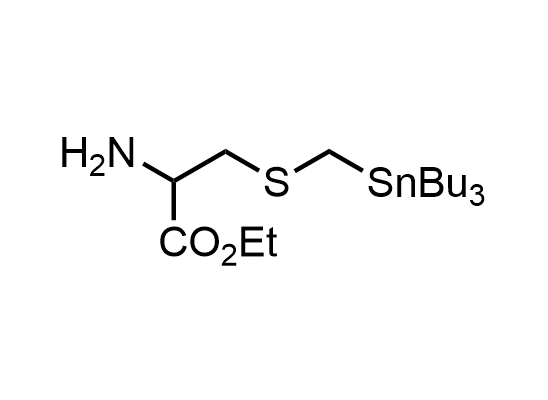
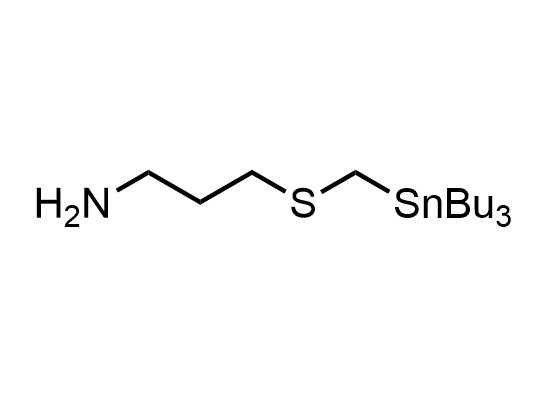
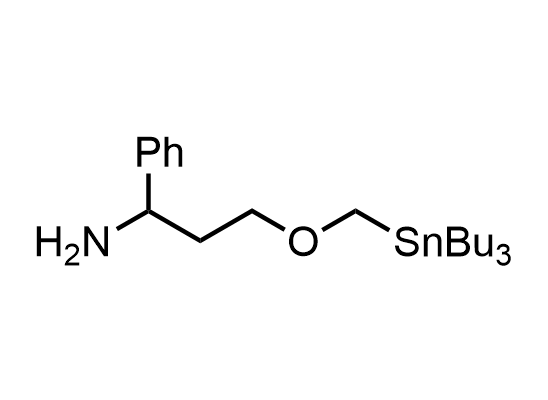
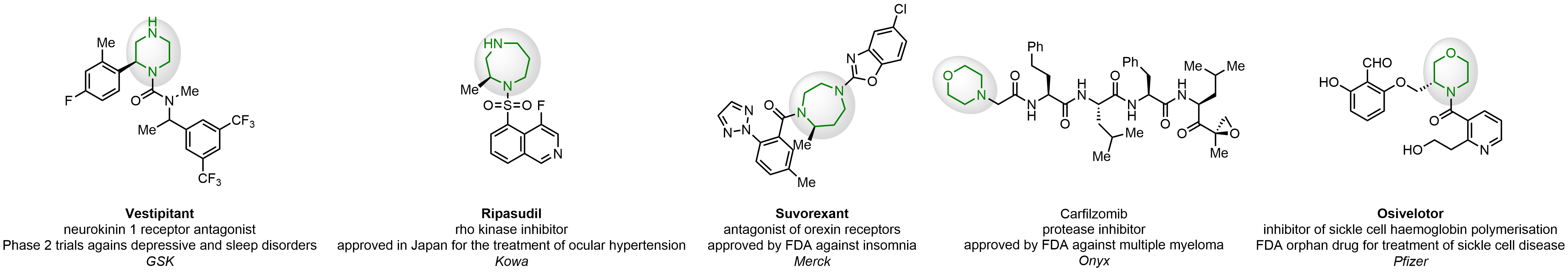
In 2013, Bode group introduced SnAP reagents for the synthesis of saturated heterocycles. Piperazines, morpholines, and their ring homologues can be prepared in two steps from carbonyl compounds and the SnAP compound. This method allows construction of heterocyclic scaffolds that are indispensable for making medicinal molecules. Try our SnAP reagents in your research!
Reaction
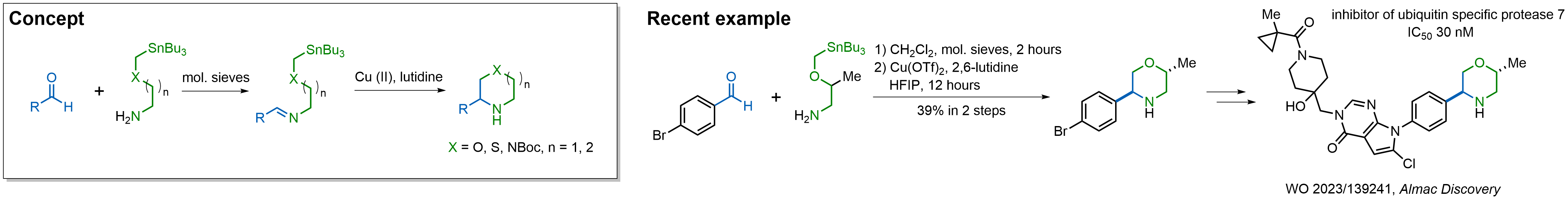
We offer
17 SnAP reagents can be ordered from our stock on 5-10 gram scale.
Pre-order
We designed a library of SnAP reagents that is ready to be synthesized upon request.