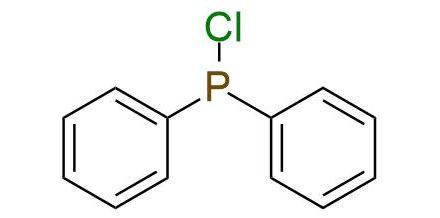
CAS 1079-66-9, Cat. No EN300-20626
Protecting group for terminal acetylenes
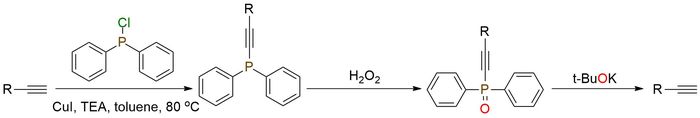
Chlorodiphenylphosphine was recently introduced as a protective group for terminal acetylenes. The protective group is stable under coupling and acidic conditions1,2. Also, the high polarity of the phosphoryl group enables easy separation of protected acetylenes from byproducts, which can be applied in the purification procedure. The protection group can be easily removed by treatment with t-BuOK. Except for this novel approach to this reagent, it can be used for chiral azide synthesis from corresponding hindered alcohols treated by diphenylchlorophosphine3. Due to its high oxygen affinity, it often allows for selectively convert alcohol to azide.
Synonyms: P,P-diphenylphosphinous chloride (ACI); phosphinous chloride, diphenyl- (6CI, 8CI, 9CI); chlorodiphenylphosphine; diphenylchlorophosphine; diphenylphosphine chloride; diphenylphosphinous chloride; diphenylphosphorus chlorid
Selected publication
-
Ph2P(O) Group for Protection of Terminal Acetylenes.
Yang X.; Matsuo D.; Suzuma Y.; Fang J.; Xu F.; Orita A.; Otera J.; Kajiyama S.; Koumura N.; Hara K. Synlett 2011, 2011 (16), 2402–2406. DOI: 10.1055/s-0030-1261223
-
Remarkable Electron-Withdrawing Effect of the Ph2P(O)-Ethynyl Group: Ph2P(O)-Ethynyl-Substituted Aryl Halides and Copper Acetylides for Tailor-Made Sonogashira Couplings.
Peng L.; Xu F.; Shinohara K.; Nishida T.; Wakamatsu K.; Orita A.; Otera J. Organic Chemistry Frontiers 2015, 2 (3), 248–252. DOI: 10.1039/C4QO00325J
-
[4‐(Acetylamino)Phenyl]Imidodisulfuryl Difluoride.
Carneiro S.; Ball N.; Lee J.; Ende C. Encyclopedia of Reagents for Organic Synthesis 2021, 1–3. DOI: 10.1002/047084289X.rn02400